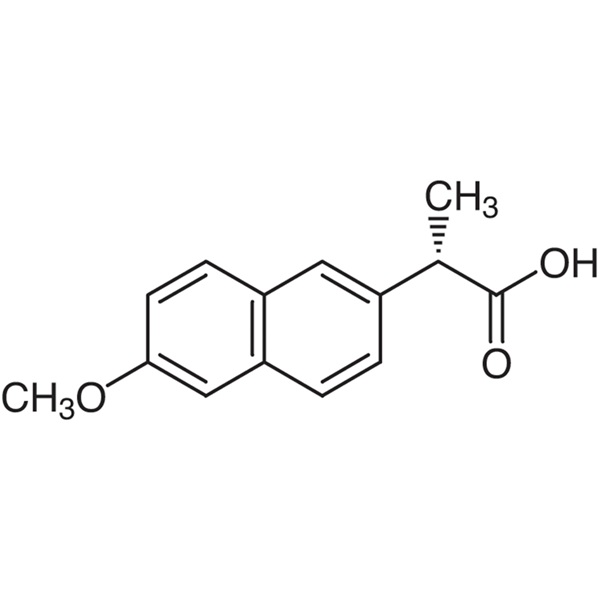
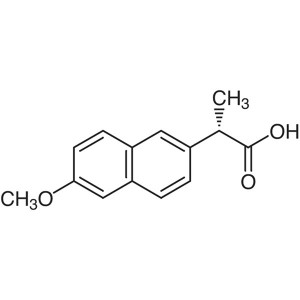
Naproxen CAS 22204-53-1 Assay 98.5~101.5% (Titration)
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer and supplier of Naproxen (CAS: 22204-53-1) with high quality. Naproxen is a non-steroidal anti-inflammatory drug. We can provide COA, worldwide delivery, small and bulk quantities available. If you are interested in this product, please send detailed information includes CAS number, product name, quantity to us. Please contact: alvin@ruifuchem.com
| Chemical Name | (S)-(+)-2-(6-Methoxy-2-Naphthyl)propionic Acid |
| Synonyms | Naproxen; DL-Naproxen; (S)-(+)-6-Methoxy-α-Methyl-2-Naphthaleneacetic Acid; (+)-6-Methoxy-alpha-Methyl-2-Naphthaleneacetic Acid; NAP |
| CAS Number | 22204-53-1 |
| Stock Status | In Stock, ProductionProduction Capacity 1000 Tons per Year |
| Molecular Formula | C14H14O3 |
| Molecular Weight | 230.26 |
| Melting Point | 154.0~158.0℃ |
| Storage & Sensitivity | Light Sensitive. Ambient Temperatures |
| Solubility | Insoluble in Water. Soluble in Alcohol |
| COA & MSDS | Available |
| Origin of Product | Shanghai, China |
| Brand | Ruifu Chemical |
| Item | Specifications | Results |
| Appearance | White to Off-White Crystalline Powder | White Crystalline Powder |
| Assay / Analysis Method | 98.5~101.5% (Neutralization Titration) | 99.61% |
| Melting Point | 154.0~158.0℃ | 154.0~155.0℃ |
| Specific Rotation [α]20/D | +83.0° to +89.5° | +85.9° |
| Loss on Drying | <0.50% (Dry it at 105℃ for 3 hours) | 0.02% |
| Residue on Ignition | <0.20% | 0.05% |
| Heavy Metals | <0.002% | <0.002% |
| Related Substances | ||
| Impurity A | <0.30% | <0.30% |
| Impurity B | <0.50% | <0.50% |
| Impurity D | <0.50% | <0.50% |
| Other Individual Impurity | <0.30% | <0.30% |
| Total Impurities | <1.00% | <1.00% |
| Infrared Spectrum | Conforms to Structure | Conforms |
| Conclusion | The product has been tested and complies with the given specifications | |
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement
Storage Condition: Keep the container tightly closed and store in a cool, dry, well-ventilated warehouse. Keep away from sunshine; avoid fire and heat sources; avoid moisture. Incompatible with strong oxidizing agents.
Shipping: Deliver to worldwide by air, by FedEx / DHL Express. Provide fast and reliable delivery.
Naproxen
C14H14O3 230.26
2-Naphthaleneacetic acid, 6-methoxy--methyl-, (S)-.
(+)-(S)-6-Methoxy--methyl-2-naphthaleneacetic acid [22204-53-1].
» Naproxen contains not less than 98.5 percent and not more than 101.5 percent of C14H14O3, calculated on the dried basis.
Packaging and storage- Preserve in tight containers.
USP Reference standards <11>-
USP Naproxen RS
Identification-
A: Infrared Absorption <197K>.
B: Ultraviolet Absorption <197U>-
Solution: 25 µg per mL.
Medium: methanol.
Absorptivities at 271 nm, calculated on the dried basis, do not differ by more than 3%.
Specific rotation <781S>: between +83.0° and +89.5°.
Test solution: 10 mg per mL, in methyl isobutyl ketone.
Loss on drying <731>- Dry it at 105℃ for 3 hours: it loses not more than 0.5% of its weight.
Heavy metals, Method II <231>: 0.002%.
Chromatographic purity- Dissolve 100 mg of Naproxen in methanol, and dilute with methanol to 5.0 mL to obtain the Test solution. Dissolve a suitable quantity of USP Naproxen RS in methanol to obtain a Standard solution having a known concentration of about 20 mg per mL. Dilute a portion of this solution quantitatively and stepwise with methanol to obtain three Comparison solutions having concentrations of 20, 60, and 100 µg per mL (0.1%, 0.3%, and 0.5% of the Standard solution), respectively. Apply separate 10-µL portions of the five solutions to the starting line of a suitable thin-layer chromatographic plate (see Chromatography 621) coated with a 0.25-mm layer of chromatographic silica gel mixture. Develop the chromatogram in a solvent system consisting of a mixture of toluene, tetrahydrofuran, and glacial acetic acid (30:3:1) until the solvent front has moved about three-fourths of the length of the plate. Remove the plate from the chamber, mark the solvent front, air-dry, and view under short-wavelength UV light: the RF value of the principal spot in the chromatogram of the Test solution corresponds to that of the Standard solution, and any other spot obtained from the Test solution does not exceed, in size or intensity, the principal spot obtained from the 100-µg-per-mL Comparison solution (0.5%), and the sum of the intensities of any secondary spots, similarly compared, does not exceed 2.0%.
Assay- Dissolve about 500 mg of Naproxen, accurately weighed, in a mixture of 75 mL of methanol and 25 mL of water that has been previously neutralized to the phenolphthalein endpoint with 0.1 N sodium hydroxide. Dissolve by gentle warming, if necessary, add phenolphthalein TS, and titrate with 0.1 N sodium hydroxide VS. Each mL of 0.1 N sodium hydroxide is equivalent to 23.03 mg of C14H14O3.
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc.
Advantages? Superior quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
Risk Codes R22 - Harmful if swallowed
R39/23/24/25 -
R23/24/25 - Toxic by inhalation, in contact with skin and if swallowed.
R11 - Highly Flammable
R36/37/38 - Irritating to eyes, respiratory system and skin.
Safety Description S36/37 - Wear suitable protective clothing and gloves.
S45 - In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
S16 - Keep away from sources of ignition.
S7 - Keep container tightly closed.
S26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
UN IDs UN 2811 6.1/PG 3
WGK Germany 3
RTECS UF5275000
HS Code 2918990090
Hazard Class 6.1(b)
Packing Group III
Naproxen (CAS: 22204-53-1) is a non-steroidal anti-inflammatory drug, it is a PG synthase inhibitor, which can inhibit prostaglandin synthetase, it has significant analgesic and antipyretic effects, oral absorption is rapid and complete, 2 to 4 hours after a dose ,plasma concentration reaches the peak, in the blood, more than 99% is bound to plasma proteins, t1/2 is 13 to 14 hours, about 95% is discharged from the urine with the prototype and metabolites. It is clinically used For the treatment of rheumatic and rheumatoid arthritis, osteoarthritis, ankylosing spondylitis, gout, arthritis, tenosynovitis.it can also be used to alleviate pain caused by musculoskeletal sprains, contusions,damages and dysmenorrhea. But it should be noted that like other non-steroidal anti-inflammatory drugs, the same serious gastrointestinal adverse reactions could occur at any time while taking naproxen during treatment, so the active gastroduodenal ulcer patients are hanged, other gastrointestinal tract disease patients should take this drug under close medical supervision. Naproxen is synthesized from 2-methoxynaphthalene and the (+)-isomer obtained by resolution with cinchonidine (61). It was introduced in the United States in 1976 and, as a generic drug, has consistently been among the more popular NSAIDs. It is marketed as the S-(+)-enantiomer, but interestingly, the sodium salt of the (-)-isomer also is on the market as Anaprox. As an inhibitor of prostaglandin biosynthesis, it is 12 times more potent than aspirin, 10 times more potent than phenylbutazone, three to four times more potent than ibuprofen, and four times times more potent than fenoprofen, but it is approximately 300 times less potent than indomethacin.