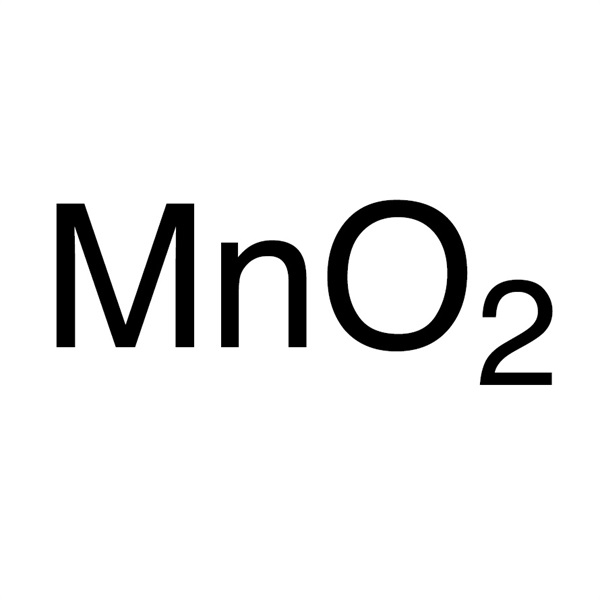
Manganese Dioxide (MnO2) CAS 1313-13-9 Purity >98.0% Hot Selling
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer and supplier of Manganese Dioxide (MnO2) (CAS: 1313-13-9) with high quality, commercial production. We can provide COA, worldwide delivery, small and bulk quantities available. Please contact: alvin@ruifuchem.com
| Chemical Name | Manganese Dioxide |
| Synonyms | MnO2; Manganese(IV) Oxide; Manganese Binoxide; Manganese Black; Manganese Superoxide |
| CAS Number | 1313-13-9 |
| Stock Status | In Stock, Production Capacity 2000MT/Year |
| Molecular Formula | MnO2 |
| Molecular Weight | 86.94 |
| Water Solubility | Insoluble in Water |
| Melting Point | 535℃ (dec.) (lit.) |
| Density | 5.02 |
| COA & MSDS | Available |
| Sample | Available |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | Black Morphous Powder or Black Orthorhombic Crystals |
| Chloride (Cl) | <0.002% |
| Sulfate (SO4) | <0.05% |
| Heavy Metals (as Pb) | <0.005% |
| Iron (Fe) | <0.02% |
| Copper (Cu) | <0.002% |
| Arsenic (As) | <0.0002% |
| Zinc (Zn) | <0.005% |
| Nickel (Ni) | <0.0005% |
| Cobalt (Co) | <0.0005% |
| Molybdenum (Mo) | <0.0005% |
| Clarity Test | Pass |
| HCl Insoluble Matter | <0.06% |
| Total Metallic Impurities | 0-20000ppm |
| Purity (MnO2) | >98.0% (Based On Trace Metals Analysis) |
| Titration by KMNO4 (Mn) | 62.0~100.0% |
| X-Ray Diffraction | Conforms to Structure |
| Physical Properties | |
| Bulk Density | 1.2~1.6g/ml |
| Particle Size (Wet Sieve) | |
| -100 Mesh | ≥99.5% |
| -200 Mesh | ≥99.0% |
| -325 Mesh | ≥95.0% |
| ElectrochemicalProperties | |
| pH (Water Method) | 5.0~7.5 |
| Test Standard | Enterprise Standard |
Package: 25kg and 50kg into a woven bag, 1000kg per pallet, or according to customer's requirement. Handle gently, prevent damage of the bags to avoid product losing and Mixing with other materials, no mix-storage with other products.
Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture

Hazard Symbols Xn - Harmful
Risk Codes 20/22 - Harmful by inhalation and if swallowed.
Safety Description 25 - Avoid contact with eyes.
UN IDs 3137
WGK Germany 1
RTECS OP0350000
TSCA Yes
HS Code 2820100000
Packing Group III
Toxicity LD50 orally in rats: >40 mmole/kg (Holbrook)
Manganese Dioxide (MnO2) (CAS: 1313-13-9), 1) Catalysts and oxidants in synthetic industry 2) Used as Colorant, achromatic agent, demineralizers, etc. in glass and enamel industries 3) Used as depolarizer for dry batteries 4) Used for manufacturing metal manganese, special alloys, ferromanganese castings, gas masks and electronic materials ferrite, etc. 5) Used in rubber industry to increase rubber viscosity. 5) Manganese Dioxide is mainly used in electrolytic zinc electrolytic lead, ceramics, chemistry industry and Battery industry. It refers to medication (Potassium Permanganate), national defense, electronical Technology, printing and dyeing, photography, ceramics, match, weld, water purification, disenfactor, oxidant, catalyst, agriculture and other industries. 6) Manganese Dioxide is an eco-friendly chemical having a high theoretical specific capacitance. 7) Manganese Dioxide has been used as a catalyst for the production of gaseous oxygen and liquid water from 30% H2O2 solution. 8) Manganese Dioxide can also be used as a catalyst in chemical experiments. 9). In the chemical industry, it produces manganese sulfate, potassium permanganate, manganese carbonate, manganese chloride, manganese nitrate and manganese oxide. It is an important raw material for chemical reagent, medicine, welding, paint and synthetic industry. 10). Used as an adsorbent for carbon monoxide in gas masks 11). It is used to manufacture metal manganese, special alloys, ferromanganese castings and electronic materials ferrite 12). It can also be used for the purification of fireworks and water, iron removal, medicine, fertilizer and fabric printing and dyeing.
Rat oral LDso:>40mmol/kg. Manganese oxide dust can cause manganese pneumoconiosis in humans. Staff should be protected. The working environment should be well ventilated. Store in a cool, dry, well-ventilated warehouse. Keep away from fire and heat source. Packaging is required to be sealed and not to be in contact with air. Moisture-proof, sunscreen. Should be stored separately from reducing agents, flammable materials, combustible materials, oxidizing agents, etc.
See chemical manganese dioxide.
Manganese oxide dust can cause human manganese pneumoconiosis. High-valent manganese oxides, regardless of the way they invade the body, have toxic effects on the brain. See metal manganese for others.
Manganese Dioxide (MnO2) is a strong oxidant, mainly used as depolarizing agent for dry batteries, decolorizing agent for glass industry, desiccant for paint ink, gas mask absorbent, match combustion aid, etc.
mainly used as depolarizing agent for dry batteries. It is a good decolorant in the glass industry, which can oxidize low-priced iron salts to high-iron salts, turning the blue-green of glass into weak yellow. Used in the electronics industry to make manganese-zinc ferrite magnetic materials. Used as a raw material for iron-manganese alloys in the steelmaking industry and a heating agent for the casting industry. Used as an absorbent for carbon monoxide in gas masks. Used in the chemical industry as an oxidant (such as purple red synthesis), a catalyst for organic synthesis, a desiccant for paints and inks. It is also used as an accelerant for the match industry, as a raw material for ceramics, enamel glaze and manganese salt. It is also used for pyrotechnics, water purification, iron removal, medicine, fertilizer and fabric printing and dyeing.
It is used in the chemical industry to produce manganese sulfate, potassium permanganate, manganese carbonate, manganese chloride, manganese nitrate, manganese monoxide, etc. Manganese sulfate is used as a fertilizer and feed additive. The pharmaceutical sector uses manganese compounds as disinfectants, oxidants, catalysts, gastric lavage agents and emetic agents. Manganese is used as a desulfurizer for dry batteries and nitrogen fertilizer production; it is also widely used in metallurgy, ceramics, glass, light industry, machinery, matches, electronics, dyes, national defense, communications, environmental protection and other departments.
See Chemical Manganese Dioxide.
It is used as a depolarizer for dry batteries, a catalyst and an oxidant for the synthesis industry, a colorant, a disinfectant, and an iron remover for the glass industry and the enamel industry. Used in the manufacture of metallic manganese, special alloys, ferromanganese castings, gas masks and electronic materials such as ferrite. In addition, it can also be used in the rubber industry to increase the viscosity of rubber.
Oxidant, special alloy steel, catalyst, desiccant, determination of sodium sulfide in cement. It is widely used in steelmaking and in making glass, ceramics, enamel, dry batteries, etc.
Used as an oxidant, also used in steelmaking, glass, ceramics, enamel, dry batteries, matches, medicine, etc.
-
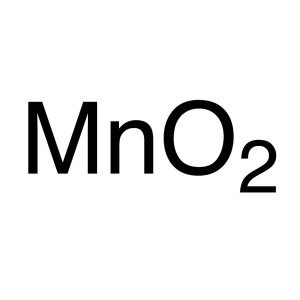
Manganese Dioxide (MnO2) CAS 1313-13-9 Purity >...
-
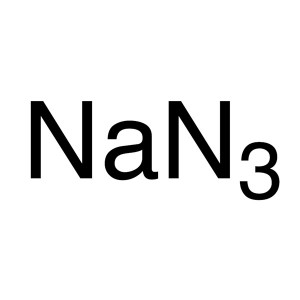
Sodium Azide CAS 26628-22-8 Purity >99.0% (T) F...
-

Sodium Amide CAS 7782-92-5 Purity >98.0% (T) Fa...
-

Cyanamide 50% in H2O CAS 420-04-2 Factory High ...
-
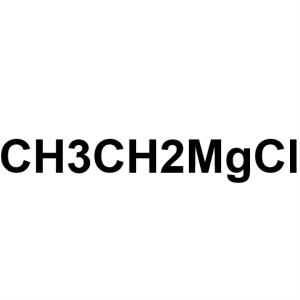
Ethylmagnesium Chloride CAS 2386-64-3 (ca. 18% ...
-
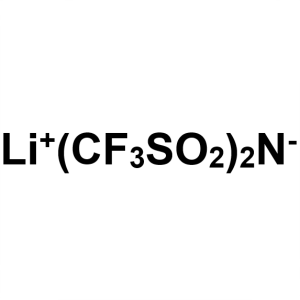
Lithium Bis(trifluoromethanesulphonyl)imide (Li...
-
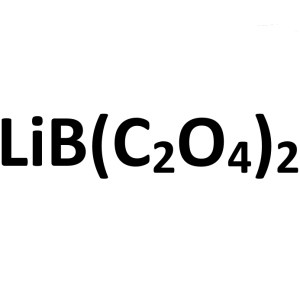
Lithium Bis(oxalate)borate (LiBOB) CAS 244761-2...
-
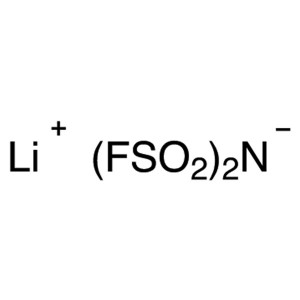
Lithium Bis(fluorosulfonyl)imide (LiFSI) CAS 17...
-
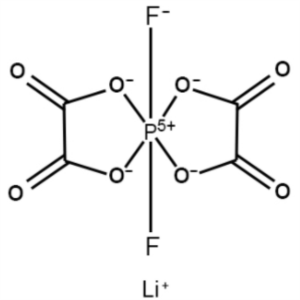
Lithium Bisoxalatodifluorophosphate (LiDODFP) C...
-

Lithium Hydroxide Anhydrous (LiOH) CAS 1310-65-...
-

Lithium Hexafluorophosphate (LiPF6) CAS 21324-4...
-

Lithium Tetrafluoroborate (LiBF4) CAS 14283-07-...
-
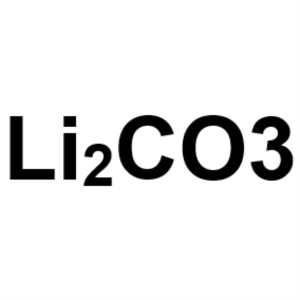
Lithium Carbonate (Li2CO3) CAS 554-13-2 Purity ...
-
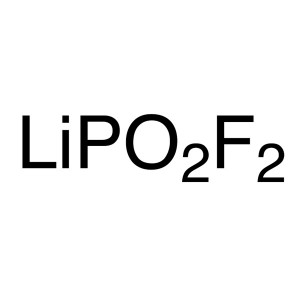
Lithium Difluorophosphate (LiPO2F2 / LiDFP) CAS...
-
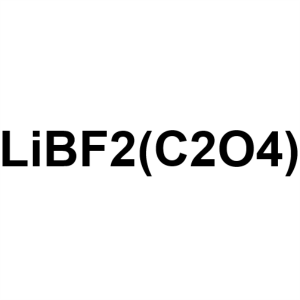
Lithium Difluoro(oxalato)borate (LiDFOB) CAS 40...
-

Ruthenium(III) Chloride Hydrate CAS 14898-67-0 ...