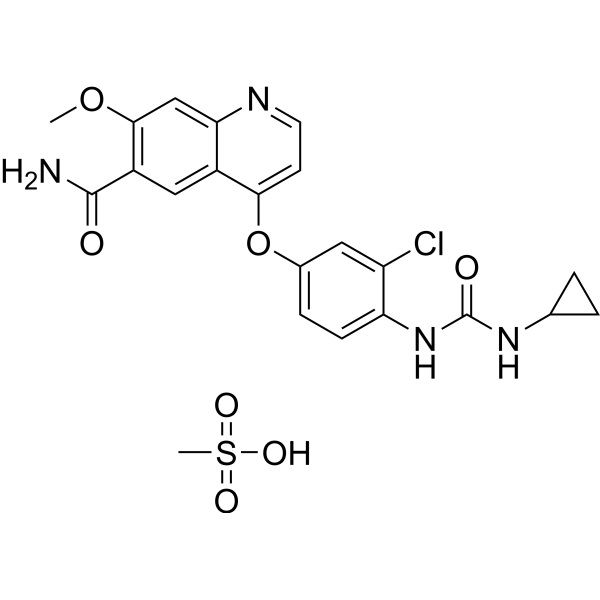
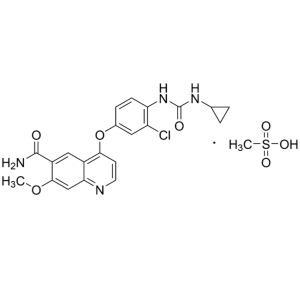
Lenvatinib Mesylate CAS 857890-39-2 Assay 98.0~102.0% Factory
Ruifu Chemical Supply Lenvatinib Mesylate Intermediates With High Purity
Lenvatinib Mesylate CAS 857890-39-2
4-Chloro-7-Methoxyquinoline-6-Carboxamide CAS 417721-36-9
Desquinolinyl Lenvatinib; 1-(2-Chloro-4-Hydroxyphenyl)-3-Cyclopropylurea CAS 796848-79-8
Methyl 7-Methoxy-4-Oxo-1,4-Dihydroquinoline-6-Carboxylate CAS 205448-65-3
Methyl 4-Amino-2-Methoxybenzoate CAS 27492-84-8
5-(Methoxymethylene)-2,2-Dimethyl-1,3-Dioxane-4,6-Dione CAS 15568-85-1
4-Amino-3-Chlorophenol CAS 17609-80-2
4-Amino-3-Chlorophenol Hydrochloride CAS 52671-64-4
Methyl 4-Chloro-7-Methoxyquinoline-6-Carboxylate CAS 205448-66-4
| Chemical Name | Lenvatinib Mesylate |
| Synonyms | 4-[3-Chloro-4-[(cyclopropylaminocarbonyl)amino]phenoxy]-7-Methoxy-6-Quinolinecarboxamide Mesylate; E 7080 Mesylate; Lenvima |
| CAS Number | 857890-39-2 |
| CAT Number | RF-PI1975 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C21H19N4O4Cl.CH4O3S |
| Molecular Weight | 522.96 |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | White to Off-White Powder or Crystals |
| Identification | By IR; By UV; By HPLC |
| Solubility | Slightly Soluble in Water, Practically Insoluble in Ethanol |
| Melting Point | 228.0~230.0℃ |
| Water Content (K.F) | <1.00% |
| Residue on Ignition | <0.10% |
| Heavy Metals | <20ppm |
| Related Substances | |
| Any Single Impurity | <0.50% |
| Total Impurities | <1.00% |
| Assay / Analysis Method | 98.0~102.0% (HPLC Basis on Drying) |
| Bulk Density | 0.40gm/ml~0.60gm/ml |
| Test Standard | Enterprise Standard |
| Usage | API |
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement
Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture


Lenvatinib Mesylate (CAS: 857890-39-2) is an oral and multi-targeted inhibitor of VEGFR1-3, FGFR1-4, PDGFR, KIT, and RET, with potent antitumor activities. Lenvatinib Mesylate is a receptor tyrosine kinase (RTK) inhibitor that has selectivity for VEGFR2. It exhibits antineoplastic activity, and has been indicated for the treatment of patients with locally recurrent or metastatic, progressive, radioactive iodine (RAI)-refractory differentiated thyroid cancer. Lenvatinib Mesylate was first approved by the U.S. Food and Drug Administration (FDA) on Feb 13, 2015, then approved by Pharmaceuticals and Medical Devices Agency of Japan (PMDA) on Mar 26, 2015, and approved by European Medicine Agency (EMA) on May 28, 2015. It was developed and marketed as Lenvima® by Eisai. Lenvatinib Mesylate is an oral multiple receptor tyrosine kinase inhibitor with a unique binding mode that selectively inhibits the kinase activities of vascular endothelial growth factor (VEGF) receptors, in addition to other proangiogenic and oncogenic pathway-related tyrosine kinases thought to be involved in tumor proliferation. It is indicated for the treatment of progressive radioiodine-refractory differentiated thyroid cancer. Lenvima is used by itself to treat differentiated thyroid cancer (DTC), a type of thyroid cancer that can no longer be treated with radioactive iodine and is progressing. LENVIMA is used along with another medicine called everolimus to treat adults with a type of kidney cancer called advanced renal cell carcinoma (RCC) after one course of treatment with another anti-cancer medicine.
-
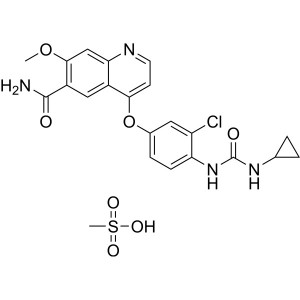
Lenvatinib Mesylate CAS 857890-39-2 Assay 98.0~...
-
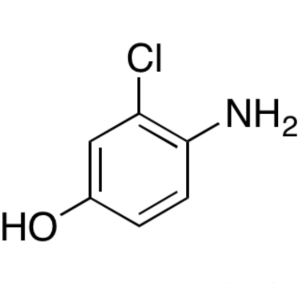
4-Amino-3-Chlorophenol CAS 17609-80-2 Lenvatini...
-
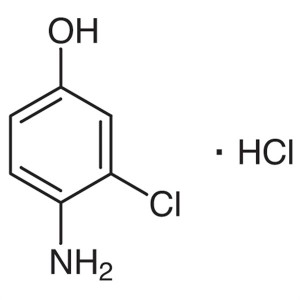
4-Amino-3-Chlorophenol Hydrochloride CAS 52671-...
-
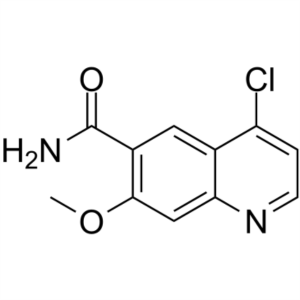
4-Chloro-7-Methoxyquinoline-6-Carboxamide CAS 4...
-
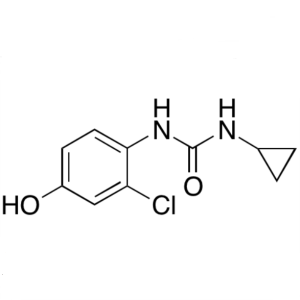
Desquinolinyl Lenvatinib CAS 796848-79-8 Purity...
-
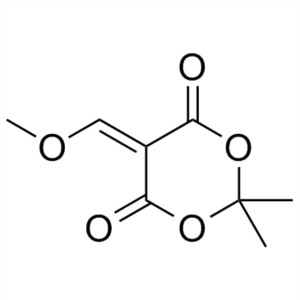
Lenvatinib Mesylate Intermediate CAS 15568-85-1...
-
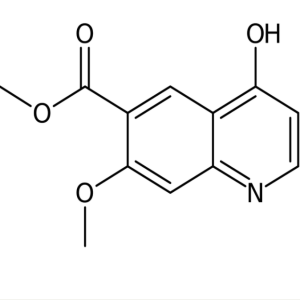
Lenvatinib Mesylate Intermediate CAS 205448-65-...
-
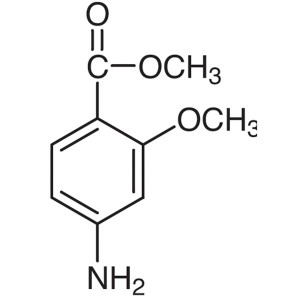
Methyl 4-Amino-2-Methoxybenzoate CAS 27492-84-8...
-
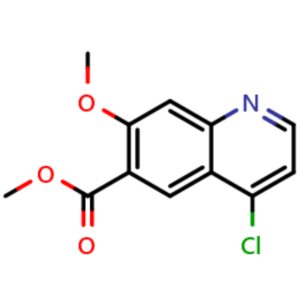
Methyl 4-Chloro-7-Methoxyquinoline-6-Carboxylat...