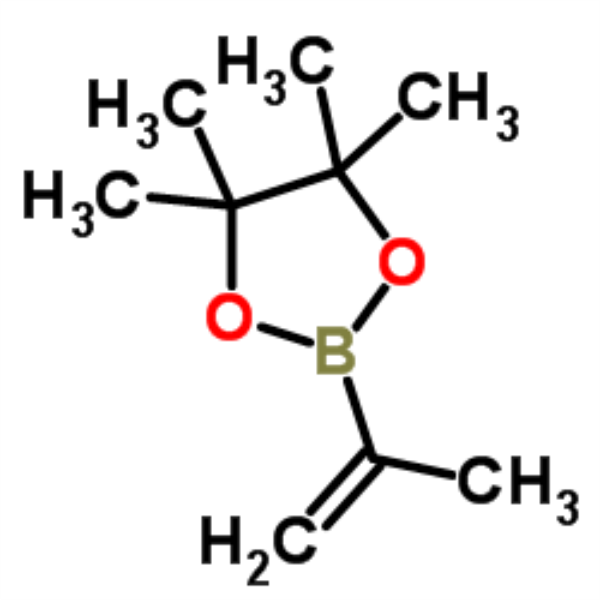
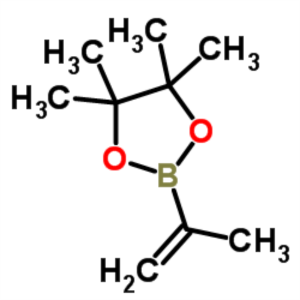
Isopropenylboronic Acid Pinacol Ester CAS 126726-62-3 Purity >99.0% (GC) Factory High Quality
Manufacturer Supply With High Quality, Commercial Production
Chemical Name: Isopropenylboronic Acid Pinacol Ester
CAS: 126726-62-3
| Chemical Name | Isopropenylboronic Acid Pinacol Ester |
| Synonyms | 4,4,5,5-tetramethyl-2-(prop-1-en-2-yl)-1,3,2-dioxaborolane; 2-(1-Methylethenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane |
| CAS Number | 126726-62-3 |
| CAT Number | RF-PI1395 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C9H17BO2 |
| Molecular Weight | 168.04 |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | Colorless to Pale Yellow Liquid |
| Purity / Analysis Method | >99.0% (GC) |
| Moisture (K.F) | ≤0.50% |
| Total Impurities | <1.00% |
| Test Standard | Enterprise Standard |
| Usage | Pharmaceutical Intermediates |
Package: Bottle, 25kg/Barrel, or according to customer's requirement.
Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture.


Isopropenylboronic Acid Pinacol Ester (CAS: 126726-62-3) is a versatile ester reagent used for palladium-catalyzed Suzuki-Miyaura cross-coupling processes, inverse-electron-demand Diels-Alder reaction, Simmons-Smith cyclopropanation reaction, polyene cyclization, stereoselective aldol reactions, Grubbs cross-metathesis reaction, intramolecular Suzuki-Miyaura reaction, Stereoselective cross-metathesis, dipolar cycloaddition, iodosulfonylation, asymmetric conjugate addition and intramolecular hydroacylation and preparation of various therapeutic kinase and enzymatic inhibitors. Isopropenylboronic Acid Pinacol Ester can be used as an intermediate in the synthesis of variety of cyclic and acyclic organic compounds. It is also shown that the α-Substituted Allyl/Croty of this compound can be used for highly Diastereoand Enantioselective allylboration of aldehydes.