Felodipine CAS 72509-76-3 ; 86189-69-7 Assay 98.0~101.0% (HPLC)
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer of Felodipine (CAS: 72509-76-3; 86189-69-7) with high quality. Ruifu Chemical offers advanced, high quality pharmaceutical intermediates and active pharmaceutical ingredients (APIs). Ruifu Chemical can provide worldwide delivery, competitive price, excellent service, small and bulk quantities available. Purchase Felodipine, Please contact: alvin@ruifuchem.com
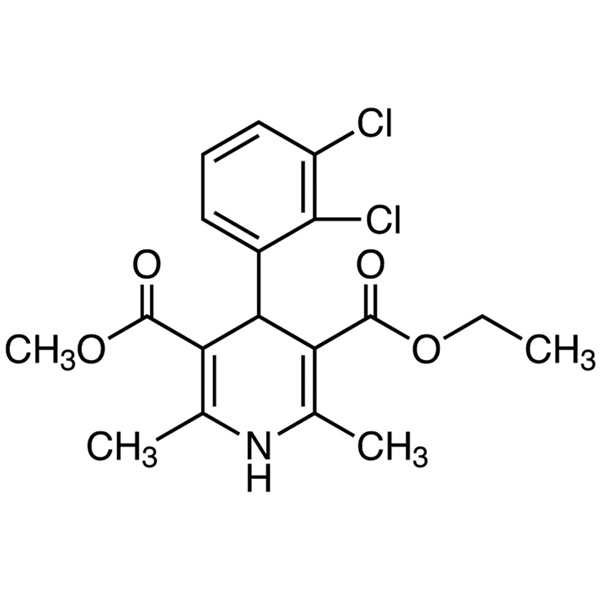
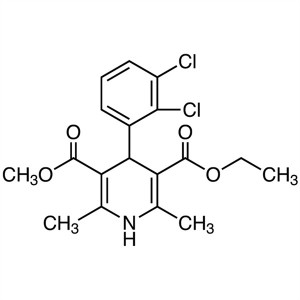
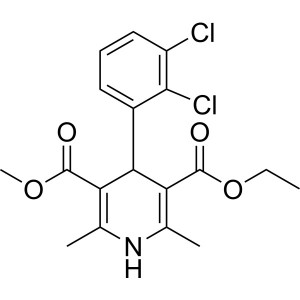
| Chemical Name | Felodipine |
| Synonyms | 4-(2,3-Dichlorophenyl)-1,4-Dihydro-2,6-Dimethyl-3,5-Pyridinedicarboxylic Acid Ethyl Methyl Ester; Ethyl Methyl 4-(2,3-Dichlorophenyl)-1,4-Dihydro-2,6-Dimethyl-3,5-Pyridinedicarboxylate; 3-Ethyl 5-Methyl 4-(2,3-dichlorophenyl)-2,6-Dimethyl-1,4-Dihydropyridine-3,5-Dicarboxylate; Spendil; CGH-869; Plendil; Renedil; Feloday |
| Stock Status | In Stock, Commercial Production |
| CAS Number | 72509-76-3; 86189-69-7 |
| Molecular Formula | C18H19Cl2NO4 |
| Molecular Weight | 384.25 g/mol |
| Melting Point | 142.0 to 146.0℃ |
| Density | 1.277g/cm3 |
| Water Solubility | Insoluble in Water |
| Solubility | Very Soluble in Acetone, Alcohol |
| COA & MSDS | Available |
| Place of Origin | Shanghai, China |
| Product Categories |
API (Active Pharmaceutical Ingredient) |
| Brand | Ruifu Chemical |
| Items | Specifications | Results |
| Appearance | White to Pale Yellow Crystalline Powder | Complies |
| Melting Point | 142.0~146.0℃ | 144.1℃ |
| Loss on Drying | <0.50% | 0.12% |
| Residue on Ignition | <0.10% | 0.05% |
| Heavy Metals (Pb) | <0.002% | <0.002% |
| Any Individual Impurity | <1.00% | <1.00% |
| Total Impurities | <1.50% | <1.50% |
| Felodipine Assay | 98.0~101.0% (HPLC) | 99.9% |
| Infrared Spectrum | Consistent with Structure | Complies |
| Conclusion | The product has been tested and complies with the given specifications | |
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Keep the container tightly closed and store in a cool, dry (2~8℃) and well-ventilated warehouse away from incompatible substances. Keep away from sunshine; avoid fire and heat sources; avoid moisture. Keep away from strong oxidizing agents.
Shipping: Deliver to worldwide by air, by FedEx / DHL Express. Provide fast and reliable delivery.
Felodipine
C18H19Cl2NO4 384.26
3,5-Pyridinedicarboxylic acid 4-(2,3-dichlorophenyl)-1,4-dihydro-2,6-dimethyl-, ethyl methyl ester, (±)-.
(±)-Ethyl methyl 4-(2,3-dichlorophenyl)-1,4-dihydro-2,6-dimethyl-3,5-pyridinedicarboxylate [72509-76-3; 86189-69-7].
» Felodipine contains not less than 98.0 percent and not more than 101.0 percent of C18H19Cl2NO4, calculated on the dried basis.
Packaging and storage- Preserve in tight, light-resistant containers, and store at controlled room temperature.
USP Reference standards <11>-
USP Felodipine RS
Color of solution- Prepare a solution in methanol having a concentration of 20 mg per mL: the absorbance, determined in a 5-cm cell at the wavelength of 440 nm in a suitable spectrophotometer, methanol being used as the blank, is not greater than 0.2.
Identification-
A: Infrared Absorption <197K>.
B: The retention time of the major peak in the chromatogram of the Assay preparation corresponds to that in the chromatogram of the Standard preparation, as obtained in the Assay.
Loss on drying <731>- Dry it at 105 for 3 hours: it loses not more than 0.5% of its weight.
Residue on ignition <281>: not more than 0.1%.
Heavy metals, Method II <231>: 0.002%.
Chromatographic purity-
Mobile phase, Standard preparation, Resolution solution, and Chromatographic system- Proceed as directed in the Assay.
Test preparation- Use the Assay preparation.
Procedure- Inject a volume (about 40 µL) of the Test preparation into the chromatograph. Allow the Test preparation to elute for not less than two times the retention time of felodipine. Record the chromatograms, and measure the areas for the impurity peaks. Calculate the percentage of each impurity in the portion of Felodipine taken by the formula:
100(ri / rs)
in which ri is the peak response for each impurity; and rs is the sum of all the responses of all the peaks: not more than 1.0% of any individual impurity is found, and the sum of all impurities is not more than 1.5%.
Assay-
Mobile phase- Dissolve 6.9 g of monobasic sodium phosphate in 400 mL of water in a 1-liter volumetric flask. Add 8.0 mL of 1 M phosphoric acid, dilute with water to volume, and mix. Prepare a filtered and degassed mixture of this solution, acetonitrile, and methanol (40:40:20). Make adjustments if necessary (see System Suitability under Chromatography <621>).
Resolution solution- Dissolve 150 mg of Felodipine in a mixture of 25 mL of tertiary butyl alcohol and 25 mL of 1 N perchloric acid, add 10 mL of 0.1 M ceric sulfate, mix, and allow to stand for 15 minutes. Add 3.5 mL of 10 N sodium hydroxide, and neutralize with 2 N sodium hydroxide. Shake the mixture with 25 mL of methylene chloride in a separator. Draw off the lower layer, and evaporate it to dryness under a stream of nitrogen on a water bath. Dissolve 10 mg of the residue (felodipine oxidation product) and 5 mg of USP Felodipine RS in Mobile phase, dilute with Mobile phase to 100 mL, and mix. Transfer 1.0 mL of the resulting solution to a 100-mL volumetric flask, dilute with Mobile phase to volume, and mix.
Standard preparation- Dissolve an accurately weighed quantity of USP Felodipine RS in Mobile phase, and quantitatively dilute with Mobile phase to obtain a solution having a known concentration of about 0.3 mg per mL. [note-Prepare this solution fresh prior to analysis.]
Assay preparation- Transfer an accurately weighed quantity of about 30 mg of Felodipine to a 100-mL volumetric flask, dissolve in and dilute with Mobile phase to volume, and mix. [note-Prepare this solution fresh prior to analysis.]
Chromatographic system (see Chromatography 621)- The liquid chromatograph is equipped with a 254-nm detector and a 4.6-mm × 15-cm column that contains 5-µm packing L1. The flow rate is about 1 mL per minute. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the capacity factor, k¢, is not less than 5.0; the column efficiency is not less than 1500 theoretical plates; and the tailing factor is not greater than 1.5. Inject 20 µL of the Resolution solution into the chromatograph, and adjust the sensitivity of the system so that the heights of the two peaks in the chromatogram are not less than 20% of recorder full scale. The resolution, R, between the first peak (felodipine oxidation product) and the second peak (felodipine) is not less than 2.5.
Procedure- Separately inject equal volumes (about 40 µL) of the Standard preparation and the Assay preparation into the chromatograph, record the chromatograms, and measure the responses for the major peaks. Calculate the quantity, in mg, of C18H19Cl2NO4 in the portion of Felodipine taken by the formula:
100C(rU / rS)
in which C is the concentration, in mg per mL, of USP Felodipine RS in the Standard preparation; and rU and rS are the peak responses obtained from the Assay preparation and the Standard preparation, respectively.
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc.
Advantages? Superior quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
| Hazard Symbols | Xn - Harmful |
| Risk Codes | 22 - Harmful if swallowed |
| Safety Description | 36 - Wear suitable protective clothing |
| UN IDs | UN 3077 9 / PGIII |
| WGK Germany | 3 |
| RTECS | US7968700 |
| HS Code | 2933 3990.99 |
Felodipine (CAS: 72509-76-3; 86189-69-7), a dihydropyridine, is a potent, vasoselective calcium channel antagonist. Felodipine lowers blood pressure (BP) by selective action on vascular smooth muscle, especially in the resistance vessels. Felodipine, an anti-hypertensive agent, induces autophagy. Felodipine can cross the blood-brain barrier. Felodipine displays high vascular selectivity; lowers arterial blood pressure without altering cardiac contractility. Antihypertensive.
Felodipine is currently indicated for use only in hypertension, either as monotherapy or in conjunction with diuretics or beta blockers.
Felodipine is used for the treatment of hypertension, ischemic heart disease, heart failure and other diseases.
Felodipine is used as a calcium ion antagonist. For mild, moderate or severe hypertension, heart failure and ischemic heart disease.
Felodipine is used for the treatment of hypertension, cardiac pain, congestive heart failure, and is effective for various degrees of hypertension.
Felodipine is a selective calcium antagonist, which mainly inhibits the inflow of extracellular calcium in arteriole smooth muscle cells, selectively expands arterioles, has no effect on the vein, does not cause orthostatic hypotension, and has no obvious effect on the myocardium Inhibition. While reducing the renal vascular resistance, Felodipine does not affect the glomerular filtration rate and creatinine clearance rate. There is no change or even a slight increase in renal blood flow, and Felodipine has natriuretic and diuretic effects. Felodipine can increase output and heart index, significantly reduce afterload, but has no obvious effect on cardiac systolic function, preload and heart rate.