Epalrestat CAS 82159-09-9 Purity >99.5% (HPLC) Factory
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer of Epalrestat (CAS: 82159-09-9) with high quality. Ruifu Chemical can provide worldwide delivery, competitive price, small and bulk quantities available. Purchase Epalrestat, Please contact: alvin@ruifuchem.com
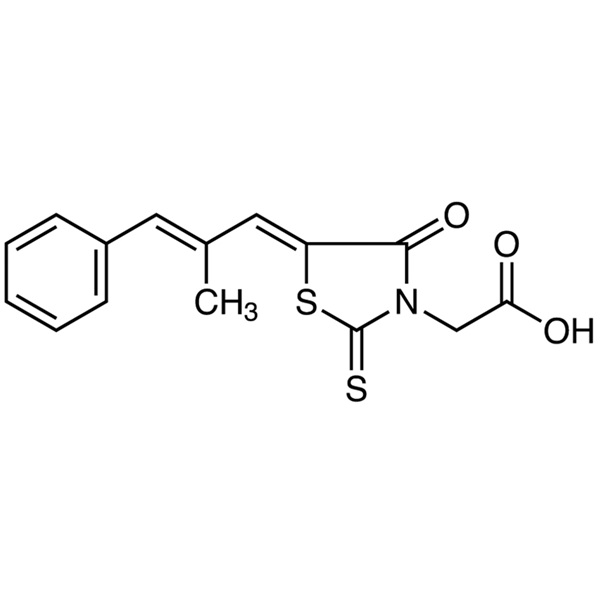
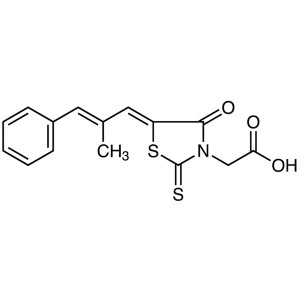
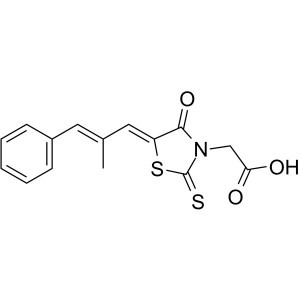
| Chemical Name | Epalrestat |
| Synonyms | Epalrest; ONO-2235; Eabeth; Kinedak; Sorbistat; (5Z)-5-[(2E)-2-Methyl-3-phenyl-2-propen-1-ylidene]-4-oxo-2-thioxo-3-Thiazolidineacetic Acid; 2-[(5Z)-5-[(E)-3-Phenyl-2-methylprop-2-enylidene]- 4-oxo-2-thioxo-3-thiazolidinyl]acetic Acid; (E,E)-2-[5-(2-Methyl-3-phenyl-prop-2-enylidene)-4-oxo-2-sulfanylidene-thiazolidin-3-yl]acetic Acid |
| Stock Status | In Stock, Mass Production |
| CAS Number | 82159-09-9 |
| Molecular Formula | C15H13NO3S2 |
| Molecular Weight | 319.39 g/mol |
| Melting Point | 222.0 to 226.0℃ |
| Density | 1.43±0.10 g/cm3 |
| Solubility | DMSO 2 mg/mL Water <1 mg/mL Ethanol <1 mg/mL |
| Storage Temp. | Cool & Dry Place (-20℃) |
| COA & MSDS | Available |
| Category | API |
| Brand | Ruifu Chemical |
| Items | Inspection Standards | Results |
| Appearance | Orange to Orange Red Crystalline Powder |
Complies |
| Identification | Identical to the HPLC retention time of standard | Pass |
| Melting Point | 222.0 to 226.0℃ | 222.4~224.3℃ |
| Loss on Drying | <0.50% | 0.04% |
| Residue on Ignition | <0.20% | 0.03% |
| Heavy Metals (Pb) | ≤20ppm | <20ppm |
| Total Impurities | ≤0.50% | 0.20% |
| Purity / Analysis Method | >99.5% (HPLC) | 99.8% |
| Infrared Spectrum | Conforms to Structure | Complies |
| Conclusion | The product has been tested & complies with the given specifications | |
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Store in sealed containers at cool and dry (-20℃) warehouse away from incompatible substances. Protect from light and moisture.
Shipping: Deliver to worldwide by FedEx / DHL Express. Provide fast and reliable delivery.
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc.
Advantages? Superior quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
UN IDs 2811
WGK Germany 3
RTECS XJ5131855
Hazard Class 6.1
Packing Group II
Epalrestat (CAS: 82159-09-9) is the second aldose reductase inhibitor to be introduced worldwide and the first to be launched in Japan. Epalrestat is indicated for the treatment of diabetic neuropathy. It is also being investigated for diabetic retinopathy and nephropathy. Epalrestat is an aldose reductase inhibitor with IC50 of 72 nM.
Epalrestat inhibits Aldose Reductase (AR) involved in the rate limiting step in the conversion of glucose to sorbitol under hyperglycemic conditions. Aldose reductase has been the target of multiple clinical investigatons to treat diabetic neuropathy and retinopathy. Epalrestat is an approved drug in Japan and India, used for the treatment of diabetic neuropathy.
Epalrestat is used to prevent, ameliorate, and treat peripheral neuropathy (numbness, pain), vibrational paresthesia, and cardiac abnormality associated with diabetes.
Epalrestat as its representative drug reduces intracellular accumulation of fructose and sorbitol by reversibly inhibiting aldose reductase activity, restores Na+-K+-ATPase and inositol activity, increases NO formation in endothelial cells, inhibits protein kinase C signalling pathway, endothelial cell adhesion, high glucose-mediated neutrophil and endothelial adhesion factor expression and reduces carboxymethyl lysine products in diabetic patients, thus treating diseases such as diabetes and its complications.