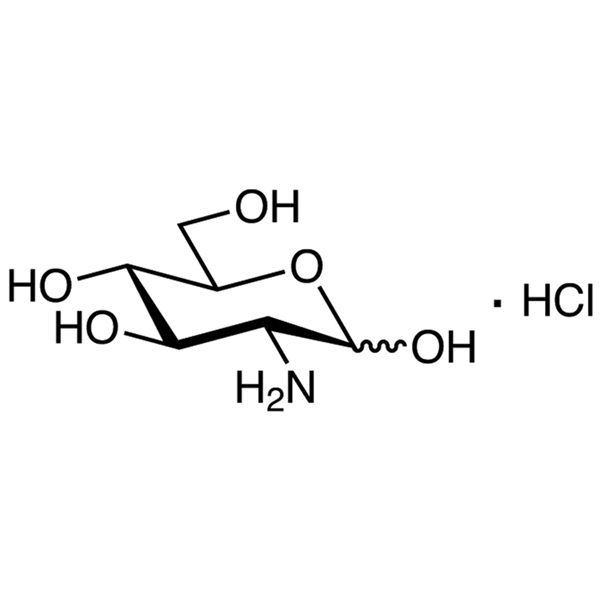
D-(+)-Glucosamine Hydrochloride CAS 66-84-2 Assay 98.0%~102.0% Factory
Ruifu Chemical is the leading manufacturer of D-(+)-Glucosamine Hydrochloride (CAS: 66-84-2) with high quality. Ruifu Chemical has 15 years experience in carbohydrate chemistry. Our R&D center is located in Shanghai and has two factories in China. Ruifu can provide worldwide delivery, competitive price, excellent service, small and bulk quantities available. Purchase D-(+)-Glucosamine Hydrochloride, Please contact: alvin@ruifuchem.com
| Chemical Name | D-(+)-Glucosamine Hydrochloride |
| Synonyms | D-Glucosamine Hydrochloride; D-Glucosamine HCl; Glucosamine Hydrochloride; 2-Amino-2-Deoxy-D-Glucopyranose Hydrochloride; alpha-D-Glucosamine Hydrochloride; Chitosamine Hydrochloride |
| Stock Status | In Stock, Production Capacity 1800 Tons per Year |
| CAS Number | 66-84-2 |
| CAS Free Base | 3416-24-8 |
| Molecular Formula | C6H13NO5·HCl |
| Molecular Weight | 215.63 g/mol |
| Melting Point | 190.0~194.0℃(dec.)(lit.) |
| Store Under Inert Gas | Store Under Inert Gas |
| Sensitive | Hygroscopic |
| Water Solubility | Soluble in Water (Clear, Colorless) |
| Stability | Stable. Incompatible with Strong Oxidizing Agents. Combustible. |
| Storage Temp. | Cool & Dry Place (2~8℃) |
| COA & MSDS | Available |
| Shelf Life | 24 Months When Properly Stored |
| Origin of Product | Shanghai, China |
| Brand | Ruifu Chemical |
| Items | Specifications | Results |
| Appearance | White Crystals Powder | White Crystals Powder |
| Identification A | Infrared Absorption | Complies |
| Identification B | Meets the requirements of the tests for chloride. | Complies |
| Identification C | The retention time of the glucosamine peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay. |
Complies |
| Assay / Analysis Method | 98.0%~102.0% (Calculated on the Dried Basis) | 99.8% |
| pH (2%, 25℃) | 3.0~5.0 | 4.12 |
| Specific Rotation [α]20/D | +70.0° ~ +73.0° (C=2.5, H2O, After 3hrs) | +71.8° |
| Loss on Drying | ≤1.00% (105℃, 2 hrs) | 0.10% |
| Residue on Ignition | ≤0.10% | 0.03% |
| Heavy Metals (Pb) | ≤10ppm | <10ppm |
| Iron (Fe) | ≤10ppm | <10ppm |
| Arsenic (AS2O3) | ≤3ppm | <1ppm |
| Sulfate (SO4) | ≤0.24% | <0.24% |
| Chloride (Cl) | 16.2%~16.7% | 16.5% |
| Total Plate Count | ≤1000 cfu/g | Complies |
| Yeast and Mold | ≤100 cfu/g | Complies |
| E. Coli. | Negative | Negative |
| Salmonella | Negative | Negative |
| Staphylococcus Aureus | Negative | Negative |
| Particle Size | 100% Through 80 Mesh | Complies |
| Solubility in H2O | Clear Colorless, 100mg/ml in Water | Pass |
| Conclusion | The product has been tested and complies with the USP35 specifications | |
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Keep the container tightly closed and store in a cool, dry (2~8℃) and well-ventilated warehouse away from incompatible substances. Avoid sunshine; avoid fire and heat sources; avoid moisture.
Shipping: Deliver to worldwide by air, by sea, by FedEx / DHL Express. Provide fast and reliable delivery.
Glucosamine Hydrochloride
C6H13NO5·HCl 215.63
d-Glucose, 2-amino-2-deoxy-, hydrochloride;
2-Amino-2-deoxy--d-glucopyranose hydrochloride [66-84-2].
DEFINITION
Glucosamine Hydrochloride contains NLT 98.0% and NMT 102.0% of glucosamine hydrochloride (C6H13NO5·HCl), calculated on the dried basis.
IDENTIFICATION
• A. Infrared Absorption <197K>
• B. Identification Tests-General, Chloride <191>: Meets the requirements
• C. The retention time of the glucosamine peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
ASSAY
• Procedure
Buffer: In a 1-L volumetric flask, dissolve 3.5 g of dibasic potassium phosphate in water. Add 0.25 mL of ammonium hydroxide, dilute with water to volume, and mix. Adjust with phosphoric acid to a pH of 7.5.
Mobile phase: Acetonitrile and Buffer (75:25)
Diluent: Acetonitrile and water (50:50)
Standard solution: 3.8 mg/mL of USP Glucosamine Hydrochloride RS in Diluent
Sample solution: 3.8 mg/mL of Glucosamine Hydrochloride in Diluent. [Note-Shake by mechanical means to aid dissolution.]
Chromatographic system
(See Chromatography <621>, System Suitability.)
Mode: LC
Detector: UV 195 nm
Column: 4.6-mm × 15-cm; 5-µm packing L8
Column temperature: 35
Flow rate: 1.5 mL/min
Injection size: 10 µL
System suitability
Sample: Standard solution
[Note-The peak for the glucosamine moiety elutes at about 10 min. The chromatogram shows a large additional peak near the void volume, due to the chloride ion. ]
Suitability requirements
Tailing factor: NMT 2.0 for the glucosamine peak
Efficiency: NLT 1500 theoretical plates
Relative standard deviation: NMT 2.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of glucosamine hydrochloride (C6H13NO5·HCl) in the portion of Glucosamine Hydrochloride taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response from the Sample solution
rS = peak response from the Standard solution
CS = concentration of USP Glucosamine Hydrochloride RS in the Standard solution (mg/mL)
CU = concentration of Glucosamine Hydrochloride in the Sample solution (mg/mL)
Acceptance criteria: 98.0%-102.0% on the dried basis
IMPURITIES
• Residue on Ignition <281>: NMT 0.1%
• Chloride and Sulfate, Sulfate 221: A 0.10-g portion shows no more sulfate than corresponds to 0.25 mL of 0.020 N sulfuric acid (NMT 0.24%).
• Arsenic, Method II <211>: NMT 3 ppm
• Heavy Metals, Method II <231>: NMT 10 ppm
SPECIFIC TESTS
• Optical Rotation, Specific Rotation <781S>: +70.0 to +73.0
Sample solution: 25 mg/mL. Measure the specific rotation 3 h after preparation.
• pH <791>
Sample solution: 20 mg/mL
Acceptance criteria: 3.0-5.0
• Loss on Drying <731>: Dry a sample at 105 for 2 h: it loses NMT 1.0% of its weight.
ADDITIONAL REQUIREMENTS
• Packaging and Storage: Preserve in tight, light-resistant containers.
• USP Reference Standards <11>
USP Glucosamine Hydrochloride RS
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc.
Advantages? Superior quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
Risk Codes R21 - Harmful in contact with skin
R36/38 - Irritating to eyes and skin.
R46 - May cause heritable genetic damage
R62 - Possible risk of impaired fertility
R63 - Possible risk of harm to the unborn child
Safety Description S24/25 - Avoid contact with skin and eyes.
S53 - Avoid exposure - obtain special instructions before use.
S36/37 - Wear suitable protective clothing and gloves.
S26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S25 - Avoid contact with eyes.
WGK Germany 2
RTECS LZ6665000
FLUKA BRAND F CODES 3-10
TSCA Yes
HS Code 29400090.90
Hazard Note Highly Flammable
D-(+)-Glucosamine Hydrochloride (CAS: 66-84-2) is a naturally occurring compound that has been used for decades in the treatment of various conditions, including arthritis, joint pain, and inflammation. It is a derivative of glucose, and is found in the human body, as well as in some foods. It is commonly used as a dietary supplement to help promote joint health and reduce inflammation.
Glucosamine Hydrochloride has been used in a variety of scientific research applications. It has been used as a substrate for the synthesis of polysaccharides and other polymers, as well as for the production of chondroitin sulfate. It has also been used in the study of the structure and properties of glycosaminoglycans, which are important components of joint cartilage. Additionally, it has been used in the study of the mechanisms of action of various drugs, including anti-inflammatory drugs.
Glucosamine Hydrochloride has been shown to have a variety of biochemical and physiological effects. It has been shown to increase the production of glycosaminoglycans, which are important components of joint cartilage. Additionally, it has been shown to reduce inflammation and promote joint health. It has also been shown to reduce pain and stiffness in joints, as well as to improve mobility.
Glucosamine Hydrochloride has been marketed as a dietary supplement for joint health for several decades. It is available in various forms, including capsules, tablets, and powders.
Glucosamine Hydrochloride has potential implications in various fields of research and industry, particularly in the development of pharmaceuticals, dietary supplements, and functional foods. It may also have applications in the production of cosmetics and personal care products. It is also a culture agent for biochemical cells.
-
D-(+)-Glucosamine Hydrochloride CAS 66-84-2 Ass...
-
D-Glucuronolactone CAS 32449-92-6 Assay 98.5%~1...
-
D-(+)-Xylose CAS 58-86-6 Purity >99.5% (HPLC) F...
-
Xylitol CAS 87-99-0 Assay 98.5~101.0% Factory H...
-
meso-Erythritol CAS 149-32-6 Assay 99.5~100.5% ...
-
D-(+)-Galactosamine Hydrochloride CAS 1772-03-8...
-
N-Methyl-D-Glucamine (Meglumine) CAS 6284-40-8 ...
-
N-Octyl-D-Glucamine CAS 23323-37-7 Purity >98.0...
-
D-(-)-Ribose CAS 50-69-1 Assay 97.0~102.0% Fact...
-
DL-Dithiothreitol (DTT) CAS 3483-12-3 Assay >98...
-
D-(+)-Galactose Anhydrous CAS 59-23-4 Assay >98...
-
D-(-)-Arabinose CAS 10323-20-3 Assay >98.5% (HP...
-
L-(+)-Arabinose CAS 5328-37-0 ; 87-72-9 Assay >...
-
D-(+)-Trehalose Dihydrate CAS 6138-23-4 Assay >...
-
D-(+)-Mannose CAS 3458-28-4 Assay >99.0% (HPLC)...
-
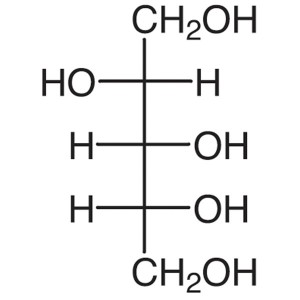
D-(+)-Arabitol CAS 488-82-4 Assay >99.0% (HPLC)...