Chloramphenicol CAS 56-75-7 Purity ≥99.0% (HPLC) High Purity
Manufacturer Supply with High Purity and Commercial Production
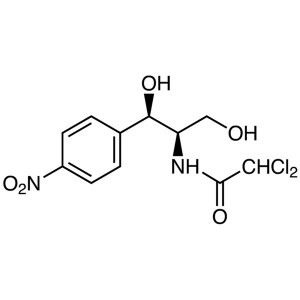
(1R,2R)-(-)-2-Amino-1-(4-nitrophenyl)-1,3-propanediol CAS 716-61-0
(1S,2S)-(+)-2-Amino-1-(4-nitrophenyl)-1,3-propanediol CAS 2964-48-9
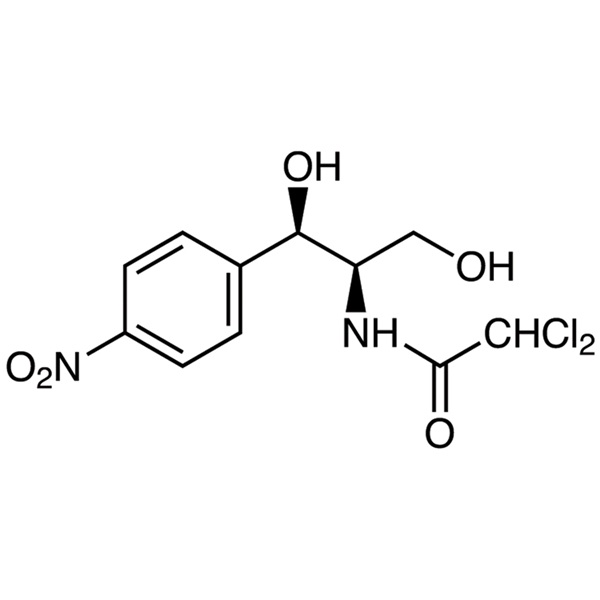
Chloramphenicol CAS 56-75-7
| Chemical Name | Chloramphenicol |
| Synonyms | D-(-)-threo-2-Dichloroacetamido-1-(4-nitrophenyl)-1,3-propanediol; 2,2-Dichloro-N-[(1R,2R)-1,3-dihydroxy-1-(4-nitrophenyl)-2-propyl]acetamide |
| CAS Number | 56-75-7 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C11H12Cl2N2O5 |
| Molecular Weight | 323.13 |
| Melting Point | 149.0 to 153.0℃ (lit.) |
| Sensitive | Light Sensitive |
| Solubility | Very Soluble in Ethanol, Methanol, Acetone. Soluble in Ether, Chloroform. Insoluble in Benzene. Practically Insoluble in Water |
| Shipping Condition | Shipped Under Ambient Temperature |
| COA & MSDS | Available |
| Origin | Shanghai, China |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | White to Light Yellow Powder |
| Identification A | Infrared Absorption |
| Identification B | The retention time of the major peak in the chromatogram of the Assay preparation corresponds to that in the chromatogram of the Standard preparation, as obtained in the Assay. |
| Specific Optical Rotation | +17.0°~+20.0° |
| Melting Point | 149.0~153.0℃ |
| Crystallinity | Meets the Requirements |
| pH | 4.5~7.5 |
| Single Impurity | ≤0.50% |
| Total Impurities | ≤1.00% |
| Heavy Metals | ≤20ppm |
| Arsenic | ≤1ppm |
| Loss on Drying | ≤0.50% (105℃, 3 hours) |
| Residue on Ignition | ≤0.10% |
| Purity / Analysis Method | ≥99.0% (HPLC) |
| Test Standard | Enterprise Standard; JP; USP |
Package: Bottle, Aluminium foil bag, 25kg/Drum, or according to customer's requirement.
Storage Condition: Store in sealed containers at cool and dry place; Protect from light, moisture.
Chloramphenicol
C11H12Cl2N2O5 323.13 [56-75-7].
Chloramphenicol contains not less than 97.0 percent and not more than 103.0 percent of C11H12Cl2N2O5.
Packaging and storage-Preserve in tight containers.
Labeling-Where it is intended for use in preparing injectable or other sterile dosage forms, the label states that it is sterile or must be subjected to further processing during the preparation of injectable or other sterile dosage forms.
USP Reference standards <11>-
USP Chloramphenicol RS Click to View Structure
USP Endotoxin RS
Identification-
A: Infrared Absorption <197K>.
B: The retention time of the major peak in the chromatogram of the Assay preparation corresponds to that in the chromatogram of the Standard preparation, as obtained in the Assay.
Melting range <741>: between 149 and 153.
Specific rotation <781S>: between +17.0 and +20.0.
Test solution: 50 mg, undried, per mL, in dehydrated alcohol.
Crystallinity <695>: meets the requirements.
Bacterial endotoxins 85-Where Chloramphenicol is intended for use in preparing injectable dosage forms, it contains not more than 0.2 USP Endotoxin Unit per mg of chloramphenicol.
Sterility 71- Where the label states that Chloramphenicol is sterile, it meets the requirements when tested as directed for Membrane Filtration under Test for Sterility of the Product to be Examined, except to use 1 g of solid specimen.
pH <791>: between 4.5 and 7.5, in an aqueous suspension containing 25 mg per mL.
Chromatographic purity-Dissolve an accurately weighed quantity of Chloramphenicol in methanol to obtain a test solution containing 10 mg per mL. Prepare a solution of USP Chloramphenicol RS in methanol containing 10 mg per mL (Standard solution A). Dilute portions of Standard solution A quantitatively with methanol to obtain Standard solution B containing 100 µg per mL and Standard solution C containing 50 µg per mL. Apply separate 20-µL portions of the test solution and Standard solutions B and C to a suitable thin-layer chromatographic plate (see Chromatography 621), coated with a 0.25-mm layer of chromatographic silica gel mixture. Develop the chromatogram in a solvent system consisting of a mixture of chloroform, methanol, and glacial acetic acid (79:14:7) until the solvent front has moved about three-fourths of the length of the plate. Remove the plate from the chamber, air-dry, and examine under short-wavelength UV light: any spot other than the principal spot obtained from the test solution does not exceed in size or intensity the principal spot obtained from Standard solution B (1%), and the sum of the impurities represented by all of the spots other than the principal spot, based on a comparison of the intensities of such spots with the intensities of the principal spots obtained from Standard solutions B and C, does not exceed 2%.
Assay-
Mobile phase-Prepare a suitable filtered mixture of water, methanol, and glacial acetic acid (55:45:0.1). Make adjustments if necessary (see System Suitability under Chromatography 621).
Standard preparation- Dissolve an accurately weighed quantity of USP Chloramphenicol RS in Mobile phase, and dilute quantitatively, and stepwise if necessary, with Mobile phase to obtain a solution having a known concentration of about 80 µg per mL. Filter a portion of this solution through a 0.5-µm or finer porosity filter, and use the clear filtrate as the Standard preparation.
Assay preparation- Transfer about 200 mg of Chloramphenicol, accurately weighed, to a 100-mL volumetric flask, add Mobile phase to volume, and mix. Transfer 4.0 mL of the resulting solution to a 100-mL volumetric flask, dilute with Mobile phase to volume, and mix. Filter a portion of this solution through a 0.5-µm or finer porosity filter, and use the clear filtrate as the Assay preparation.
Chromatographic system (see Chromatography <621>)-The liquid chromatograph is equipped with a 280-nm detector and a 4.6-mm × 10-cm column that contains 5-µm packing L1. The flow rate is about 1 mL per minute. Chromatograph the Standard preparation, and record the peak responses as directed under Procedure: the column efficiency determined from the analyte peak is not less than 1800 theoretical plates, the tailing factor is not more than 2.0, and the relative standard deviation for replicate injections is not more than 1.0%.
Procedure-[note-Use peak heights where peak responses are indicated. ] Separately inject equal volumes (about 10 µL) of the Standard preparation and the Assay preparation into the chromatograph, record the chromatograms, and measure the responses for the major peaks. Calculate the quantity, in mg, of C11H12Cl2N2O5 in the portion of Chloramphenicol taken by the formula:
2.5C(rU / rS)
in which C is the concentration, in µg per mL, of USP Chloramphenicol RS in the Standard preparation, and rU and rS are the peak responses obtained from the Assay preparation and the Standard preparation, respectively.
Chloramphenicol
C11H12Cl2N2O5: 323.13
2,2-Dichloro-N-[(1R,2R)-1,3-dihydroxy-1-(4-nitrophenyl)propan-2-yl]acetamide [56-75-7]
Chloramphenicol contains not less than 980 mg (potency) and not more than 1020 mg (potency) per mg, calculated on the dried basis. The potency of Chloramphenicol is expressed as mass (potency) of chloramphenicol (C11H12Cl2N2O5).
Description Chloramphenicol occurs as white to yellowish white, crystals or crystalline powder.
It is freely soluble in methanol and in ethanol (99.5), and slightly soluble in water.
Identification (1) Determine the absorption spectrum of the sample solution obtained in the Assay as directed under Ultraviolet-visible Spectrophotometry <2.24>, and compare the spectrum with the Reference Spectrum or the spectrum of a solution of Chloramphenicol RS prepared in the same manner as the sample solution: both spectra exhibit similar intensities of absorption at the same wavelengths.
(2) Determine the infrared absorption spectrum of Chloramphenicol as directed in the potassium bromide disk method under Infrared Spectrophotometry <2.25>, and compare the spectrum with the Reference Spectrum or the spectrum of Chloramphenicol RS: both spectra exhibit similar intensities of absorption at the same wave numbers.
Optical rotation <2.49> [a]20D: +18.5~+21.5℃ (1.25 g, ethanol (99.5), 25 mL, 100 mm).
Melting point <2.60> 150~155℃
Purity (1) Heavy metals <1.07>-Proceed with 1.0 g of Chloramphenicol according to Method 2, and perform the test. Prepare the control solution with 2.5 mL of Standard Lead Solution (not more than 25 ppm).
(2) Arsenic <1.11>-Prepare the test solution with 2.0 g of Chloramphenicol according to Method 4, and perform the test (not more than 1 ppm).
(3) Related substances-Dissolve 0.10 g of Chloramphenicol in 10 mL of methanol, and use this solution as the sample solution. Pipet 1 mL of the sample solution, add methanol to make exactly 100 mL, and use this solution as the standard solution (1). Pipet 10 mL of the standard solution (1), add methanol to make exactly 20 mL, and use this solution as the standard solution (2). Perform the test with these solutions as directed under Thin-layer Chromatography <2.03>. Spot 20 mL each of the sample solution and standard solutions (1) and (2) on a plate of silica gel with fluorescent indicator for thin-layer chromatography, develop the plate with a mixture of chloroform, methanol and acetic acid (100) (79:14:7) to a distance of about 15 cm, and air-dry the plate. Examine under ultraviolet light (main wavelength: 254 nm): the spots other than the principal spot and the spot on the original obtained from the sample solution are not more intense than the spot obtained from the standard solution (1), and the total amunt of these spots from the sample solution is not more than 2.0%.
Loss on drying <2.41> Not more than 0.5% (1 g, 105℃, 3 hours).
Residue on ignition <2.44> Not more than 0.1%(1 g).
Assay Weigh accurately an amount of Chloramphenicol and Chloramphenicol RS, equivalent to about 0.1 g (potency), dissolve each in 20 mL of methanol, and add water to make exactly 100 mL. Pipet 20 mL each of these solutions, and add water to make exactly 100 mL. Pipet 10 mL each of these solutions, add water to make exactly 100 mL, and use these solutions as the sample solution and standard solution. Determine the absorbances, AT and AS, at 278 nm of the sample solution and standard solution as directed under Ultraviolet-visible Spectrophotometry <2.24>.
Amount [mg (potency)] of chloramphenicol (C11H12Cl2N2O5)
= MS × AT/AS × 1000
MS: Amount [mg (potency)] of Chloramphenicol RS
taken
Containers and storage Containers-Tight containers.

Risk Codes R45 - May cause cancer
R11 - Highly Flammable
R39/23/24/25 -
R23/24/25 - Toxic by inhalation, in contact with skin and if swallowed.
Safety Description S53 - Avoid exposure - obtain special instructions before use.
S45 - In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
S16 - Keep away from sources of ignition.
S36/37 - Wear suitable protective clothing and gloves.
UN IDs 2811
WGK Germany 3
RTECS AB6825000
FLUKA BRAND F CODES 3-10
TSCA Yes
HS Code 2941400000
Hazard Class 3
Toxicity LD50 oral in rat: 2500mg/kg
P501: Dispose of contents/ container to an approved waste disposal plant.
P260: Do not breathe dust/ fume/ gas/ mist/ vapors/ spray.
P270: Do not eat, drink or smoke when using this product.
P202: Do not handle until all safety precautions have been read and understood.
P201: Obtain special instructions before use.
P264: Wash skin thoroughly after handling.
P280: Wear protective gloves/ protective clothing/ eye protection/ face protection.
P308 + P313: IF exposed or concerned: Get medical advice/ attention.
P405: Store locked up.
Shanghai Ruifu Chemical Co., Ltd. is the leading supplier of Chloramphenicol (CAS: 56-75-7) with high quality. Chloramphenicol is a broad-spectrum antibacterial antibiotic, which is the first choice for the treatment of typhoid and paratyphoid, secondly, it is used for the treatment of various infectious diseases caused by sensitive microorganisms. Due to serious adverse reactions are now used less and less. The antibacterial spectrum, function and use are the same as chloramphenicol for the treatment of Salmonella Typhi, Shigella, Escherichia coli, influenza, Brucella, pneumonia infections caused by cocci and other antibiotics anti-infective drugs. Chloramphenicol is mainly used to treat urinary tract infection, Pneumonia, abdominal infection and septicemia caused by sensitive bacteria, as well as external eye drops and ear drops.
UN3249 Medicine, solid, toxic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials. UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1- Poisonous materials, Technical Name Required.