Vardenafil Intermediate CAS 224789-21-3 Purity >99.0% (HPLC)
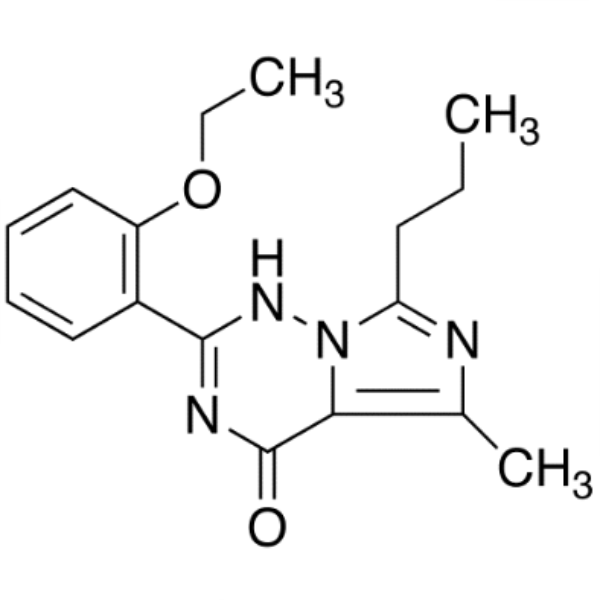
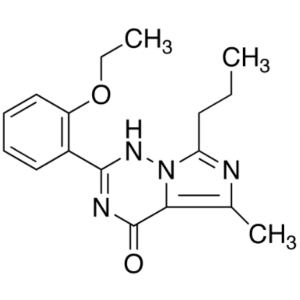
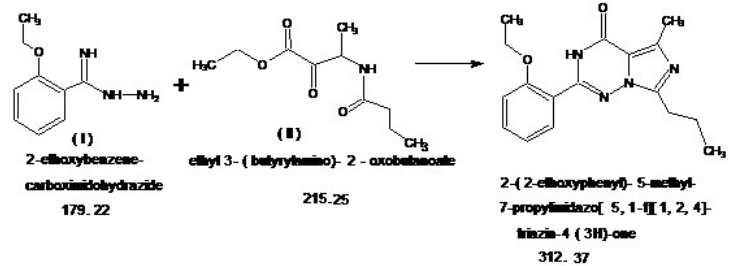
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer of 2-(2-Ethoxyphenyl)-5-Methyl-7-Propylimidazo[5,1-F][1,2,4]triazin-4(3H)-one (CAS: 224789-21-3) with high quality, intermediate of Vardenafil. Ruifu Chemical can provide worldwide delivery, competitive price, excellent service, small and bulk quantities available. Purchase Vardenafil Intermediates, Please contact: alvin@ruifuchem.com
| Chemical Name | 2-(2-Ethoxyphenyl)-5-Methyl-7-Propylimidazo[5,1-F][1,2,4]triazin-4(3H)-one |
| Synonyms | Vardenafil Dessulfonyl Impurity; 2-(2-Ethoxyphenyl)-5-Methyl-7-Propyl-3H-Imidazo[5,1-F][1,2,4]triazin-4-one; Vardenafil Propyl Imidazotriazinone Impurity; Vardenafil Related Compound 1 |
| Stock Status | In Stock, Production Capacity 2 Tons per Month |
| CAS Number | 224789-21-3 |
| Molecular Formula | C17H20N4O2 |
| Molecular Weight | 312.37 g/mol |
| Melting Point | 142.0~148.0℃ |
| Density | 1.26±0.10 g/cm3 |
| Long-Term Storage | Cool & Dry Place (2~8℃) |
| COA & MSDS | Available |
| Origin | Shanghai, China |
| Brand | Ruifu Chemical |
| Items | Specifications | Results |
| Appearance | Off-White to Pale Yellow Powder | Off-White Powder |
| Melting Point | 142.0~148.0℃ | 146.0~147.0℃ |
| Loss on Drying | <1.00% (102℃, 3h) | 0.23% |
| Heavy Metals (Pb) | ≤20ppm | <20ppm |
| Purity / Analysis Method | >99.0% (HPLC) | 99.79% |
| 1H NMR Spectrum | Consistent with Structure | Complies |
| Conclusion | The product has been tested and complies with the given specifications | |
| Application | Intermediate of Vardenafil / Vardenafil Hydrochloride | |
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Keep the container tightly closed and store in a cool, dry (2~8℃) and well-ventilated warehouse away from incompatible substances. Protect from sunlight and moisture.
Shipping: Deliver to worldwide by air, by FedEx / DHL Express. Provide fast and reliable delivery.
1. Appearance: Put the sample on white paper and observe the appearance, off-white to pale yellow powder.
2. Analysis:
2.1 Melting Point determination according to law (Chinese Pharmacopoeia 2010 Edition II Appendix ⅥC) Melting point is 142.0~148.0℃.
2.2 Loss on Drying: Determination according to law (Appendix ⅧL, Part II, 2010 Edition of Chinese Pharmacopoeia). Precision weighing appropriate amount of this product, dry at 102℃ for 3 hours, weight loss shall not be more than 1.00%.
2.3 Heavy Metals: Determination according to law (People's Republic of China Pharmacopoeia 2010 Edition, Part II Appendix ⅧH) Determination by the second method.
Precision weigh 1.0g of the test product, slowly burning until completely carbonized, cool, add 0.5-1.0ml sulfuric acid, make it moist, heat at low temperature until the sulfuric acid is exhausted, add 0.5ml nitric acid, steam dry, until the nitrogen oxide vapor is exhausted, cool, hot at 500-600℃ to completely carbonized, cool, add 2ml hydrochloric acid, After drying in a water bath, add 15ml water, drop the ammonia test solution until it becomes neutral to the phenolphthalein indicator solution, add 2ml acetate buffer (ph3.5), and slightly heat dissolve, move it to the Nachner colorimetric tube, and dilute it into 25ml with water. Take another reagent for preparing the test solution, and steam it in a porcelain dish, add 2ml acetate buffer (PH3.5) and 15ml water to slightly heat After dissolution, move the Nath colorimetric tube, add a certain amount of standard lead solution, and then dilute with water to 25ml. Colorimetric experiment is carried out between test solution and standard solution to obtain. Standard solution preparation according to (People's Republic of China Pharmacopoeia 2010 edition II Appendix Ⅷ Heavy metal test method) the first method. Heavy metals must not exceed 20 parts per million.
3. Purity: According to high performance liquid chromatography, determined according to law (Chinese Pharmacopoeia 2010 edition II Appendix VD).
Chromatographic conditions:
Column: C18, 200mm×4.6mm, 5μm;
Mobile phase: Phosphate buffer (weigh 2.72g potassium dihydrogen phosphate, add water to dissolve and dilute to 1000ml, adjust pH=3.5) : acetonitrile: = 35:65;
Detection wavelength: 254nm;
Flow rate: 1.0ml/min.
Determination method: Take about 30mg of this product, weigh it accurately, dilute it with mobile phase to make the concentration of about 0.3mg/ml, take 20μl into the liquid chromatograph, record the chromatogram. According to area normalization method, the purity is not less than 99.0%.
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc.
Advantages? Superior quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
2-(2-Ethoxyphenyl)-5-Methyl-7-Propylimidazo[5,1-F][1,2,4]triazin-4(3H)-one (CAS: 224789-21-3) is an intermediate of Vardenafil (CAS: 224785-90-4) / Vardenafil Hydrochloride (CAS: 224785-91-5). Vardenafil is a new PDE5 inhibitor launched for oral treatment of male erectile dysfunction and it has significant structural similarity with sildenafil (Viagra), which was the first PDE5 inhibitor introduced in 1998 for this indication.
Vardenafil was the second agent to be marketed and had the advantage that its onset time was not reduced by taking the medication on a full stomach . It is 30 times more potent as an inhibitor of PDE5 (mean IC50, 3.9 nM) than sildenafil and 10 times more potent than tadalafil, with a greater selectivity (>1,000 times) for human PDE5 than for human PDE2, PDE3, and PDE4 and moderate selectivity (>80 times) for PDE1. The PDE inhibitory selectivity and both the in vitro and in vivo potency of the new PDE5 inhibitor vardenafil. Vardenafil specifically inhibited the hydrolysis of cGMP by PDE5, with an IC50 of 0.7 nM (sildenafil 6.6 nM). The IC50 of vardenafil for PDE1 was 180 nM, for PDE6 11 nM, and for PDE2, PDE3 and PDE4 more than 1,000 nM.