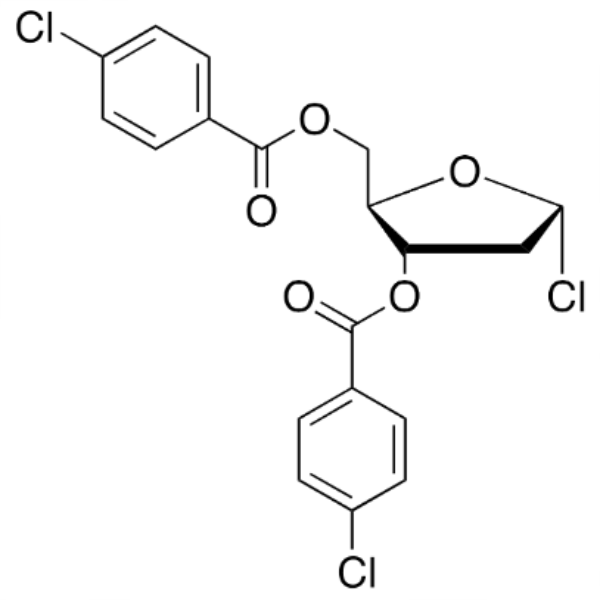
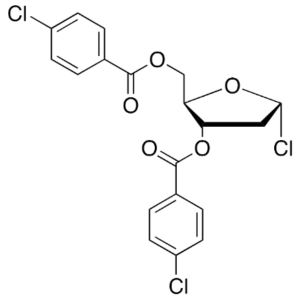
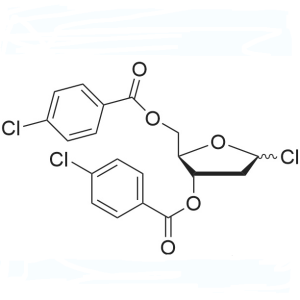
1-Chloro-3,5-Di-(4-Chlorobenzoyl)-2-Deoxy-D-Ribose CAS 3601-90-9; 21740-23-8 Assay >90.0% (HPLC) Trifluridine Decitabine Intermediate
Ruifu Chemical is the leading manufacturer of 1-Chloro-3,5-Di-(4-Chlorobenzoyl)-2-Deoxy-D-Ribose (CAS: 3601-90-9; 21740-23-8) with high quality, intermediate of Trifluridine (CAS: 70-00-8) and Decitabine (CAS: 2353-33-5). Ruifu Chemical can provide worldwide delivery, competitive price, excellent service, small and bulk quantities available. Purchase Trifluridine and Decitabine Intermediates, Please contact: alvin@ruifuchem.com
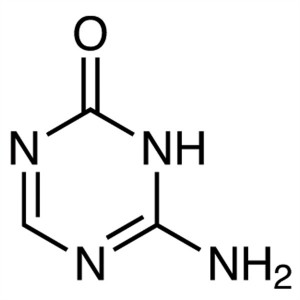
| Trifluridine | CAS 70-00-8 |
| 1-Chloro-3,5-Di-O-Toluoyl-2-Deoxy-D-Ribofuranose | CAS 3601-89-6 |
| 1-Chloro-3,5-Di-(4-Chlorobenzoyl)-2-Deoxy-D-Ribose | CAS 3601-90-9; 21740-23-8 |
| Chemical Name | 1-Chloro-3,5-Di-(4-Chlorobenzoyl)-2-Deoxy-D-Ribose |
| Synonyms | 1-Chloro-3,5-Di-(p-Chlorobenzoyl)-2-Deoxy-D-Rribofuranose; 3,5-O-Bis(p-Chlorobenzoyl)-2-Deoxy-α-D-Ribofuranosyl Chloride; 3,5-Bis-o-(4-Chlorobenzoyl)-2-Deoxypentofuranosyl Chloride; 1-Cl-3,5-Bis-(4-Cl-bz)-2-Deoxy-D-Ribofuranose; 1-Chloro-2-Deoxy-3,5-O-Bis(p-Chlorobenzoyl)-α-D-Erythro-Pentofuranose; 3,5-Bis(4-Chlorobenzoyl)-2-Deoxy-alpha-D-Ribofuranosyl Chloride |
| Stock Status | In Stock, Commercial Production |
| CAS Number | 3601-90-9 |
| Related CAS Number | 21740-23-8 |
| Molecular Formula | C19H15Cl3O5 |
| Molecular Weight | 429.67 g/mol |
| Melting Point | 115.0~125.0℃ |
| Density | 1.46±0.10 g/cm3 |
| Storage Temp. | Cool & Dry Place (0~8℃) |
| COA & MSDS | Available |
| Origin of Product | Shanghai, China |
| Product Categories |
Pharmaceutical Intermediates |
| Brand | Ruifu Chemical |
| Items | Specifications | Results |
| Appearance | White or Almost White Powder | Complies |
| Melting Point | 115.0~125.0℃ | 115.4~118.0℃ |
| Loss on Drying | <1.00% | 0.36% |
| Residue on Ignition | <0.10% | 0.08% |
| Heavy Metals (Pb) | <20ppm | <20ppm |
| Impurity 1 | <2.50% | Complies |
| Impurity 2 | <5.00% | Complies |
| Impurity 3 | <1.50% | Complies |
| Other Individual Impurity | <0.50% | Complies |
| Total Impurities | <10.0% | Complies |
| Assay / Analysis Method | >90.0% (HPLC) | 92.6% |
| Infrared Spectrum | Consistent with Structure | Complies |
| Conclusion | The product has been tested and complies with the given specifications | |
| Application | Intermediate of Trifluridine (CAS: 70-00-8) and Decitabine (CAS: 2353-33-5) | |
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Keep the container tightly closed and store in a cool, dry (0~8℃), well-ventilated warehouse away from incompatible substances. Keep away from sunshine; avoid fire and heat sources; avoid moisture.
Shipping: Deliver to worldwide by air, by FedEx / DHL Express. Provide fast and reliable delivery.
1-Chloro-3,5-Di-(4-Chlorobenzoyl)-2-Deoxy-D-Ribose (CAS: 21740-23-8) Method of Analysis
Properties: This product is white or off-white powder.
Identification: The infrared spectrum of this product should be consistent with that of the reference product (Appendix IV C of the second part of the 2010 edition of Chinese Pharmacopoeia).
Analysis: Related substances. Take an appropriate amount of this product, weigh it accurately, dissolve it with acetonitrile-water (80:20) and dilute it quantitatively to make a solution containing about 0.5mg in every 1ml as the test product solution. According to the chromatographic conditions under the content determination item, the sample solution of 10μl was precisely measured, injected into the high performance liquid chromatograph (HPLC) equipment, and the chromatogram was recorded. If there is an impurity peak in the solution chromatogram of the test product, the impurity 1 (relative retention time is about 0.14) shall not exceed 2.5%, impurity 2 (relative retention time is about 2.1) shall not exceed 5.0%, impurity 3 (relative retention time is about 2.3) shall not exceed 1.5%, and other single impurity shall not exceed 0.5%, according to the area normalization method. Total impurities must not exceed 10.0%
Loss on drying: Take an appropriate amount of this product, phosphorus pentoxide as a desiccant, drying at 40℃ vacuum to constant weight, weight loss should not exceed 1.0% (Chinese Pharmacopoeia 2010 edition, Part II Appendix Ⅷ L).
Assay: According to high performance liquid chromatography (Chinese Pharmacopoeia 2010, Part II Appendix V D) determination.
Chromatographic conditions and system suitability test: Octadecylsilane bonded silica gel was used as filler (specification: 4.6×250mm, 5μm), 0.01mol/L potassium dihydrogen phosphate solution (pH adjusted to 6.5 by 1mol/L potassium hydroxide solution) -acetonitrile (90:10) as mobile phase A, acetonitrile as mobile phase B, and linear gradient elution was performed according to the following table. The detection wavelength was 205nm; The column temperature was 30℃, the flow rate was 1ml per minute, and the sample chamber temperature was 2~8℃. The separation degree between the main peak and adjacent impurity peaks should meet the requirements.
Time (min) Mobile phase A (%) Mobile phase B (%)
0 50 50
20 50 50
40 33 67
55 33 67
56 50 50
65 50 50
Measurement: Take an appropriate amount of this product, weigh it accurately, dissolve it with acetonitrile-water (80:20) and dilute it quantitatively to make a solution containing about 0.5mg in every 1ml as the test product solution. The sample solution of 10μl was accurately measured, injected into the high performance liquid chromatograph (HPLC) equipment, and the chromatogram was recorded. According to the area normalization method, the main peak content should not be less than 90.0%.
Storage: Dark, sealed, stored in a cold place.
Validity: 12 months.
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc.
Advantages? Superior quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
1-Chloro-3,5-Di-(4-Chlorobenzoyl)-2-Deoxy-D-Ribose (CAS: 3601-90-9; 21740-23-8), intermediate of Trifluridine (CAS: 70-00-8) and Decitabine (CAS: 2353-33-5).
Trifluridine, a fluorinated pyrimidine nucleoside, is an anti-herpesvirus agent and an antitumor antimetabolite agent. It is an analog of thymidine which inhibits thymidylate synthase possesses antiviral and anticancer activity. After phosphorylation by thymidine kinase, it is incorporated into DNA where it induces DNA-damage and interferes with repair enzymes. Enhances frame shift insertion and deletion in CRISPR genome editing in pluripotent stem cells.
Decitabine, has been launched for the treatment of myelodysplastic syndromes (MDS). MDS are a set of hematologic disorders affecting the bone marrow that result in ineffective formation and development of blood cells. Furthermore, patients with MDS have a high risk of progressing to acute myeloid leukemia (AML). Traditional treatments include blood transfusions, hematopoietic growth factors, and prophylactic antibiotics, but these measures merely improve the quality of life with questionable effects on disease modification. While stem-cell transplantation is an aggressive, potentially curative approach, the advanced age or the other complicating health issues of most patients preclude them from considering this option. Recent advances in the underlying etiology of MDS, however, have led to the development of a new class of compounds known as ''demethylating agents''. Decitabine follows the successful introduction of the first DNA methyltransferase inhibitor azacitidine.