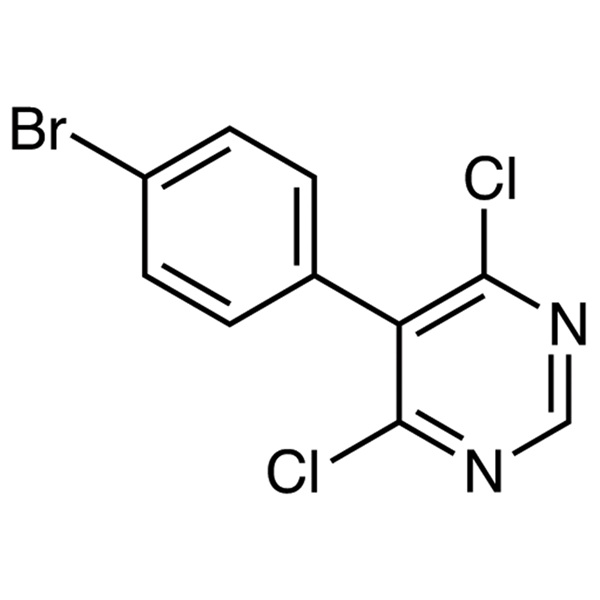
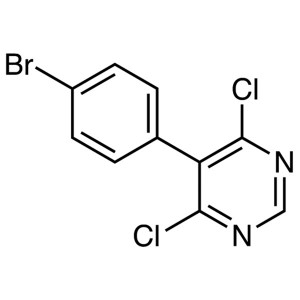
5-(4-Bromophenyl)-4,6-Dichloropyrimidine CAS 146533-41-7 Macitentan Intermediate Purity ≥99.0% (HPLC) Factory
| Chemical Name | 5-(4-Bromophenyl)-4,6-Dichloropyrimidine |
| Synonyms | Macitentan Intermediate 1; Macitentan Impurity 26 |
| CAS Number | 146533-41-7 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C10H5BrCl2N2 |
| Molecular Weight | 303.97 |
| Melting Point | 98.0 to 102.0℃ |
| Density | 1.677±0.06 g/cm3 |
| COA & MSDS | Available |
| Place of Origin | Shanghai, China |
| Product Categories | Pharmaceutical Intermediates |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | White to Off-White Powder |
| Purity / Analysis Method | ≥99.0% (HPLC) |
| Impurity A | ≤0.50% (5-(4-Bromophenyl)-6-Chloropyrimidine-4-ol) |
| Impurity B | ≤0.10% (4,6-Dichloro-5-(4-Chlorophenyl)pyrimidine) |
| Any Unspecified Impurity | ≤0.30% |
| Total Impurities | ≤1.00% |
| Loss on Drying | ≤0.50% |
| Water Content | ≤0.50% |
| Infrared Spectrum | Conforms to Structure |
| NMR Spectrum | Conforms to Structure |
| Test Standard | Enterprise Standard |
| Usage | Intermediate of Macitentan (CAS: 441798-33-0) |
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture.

5-(4-Bromophenyl)-4,6-Dichloropyrimidine (CAS: 146533-41-7) is a key intermediate used in the synthesis of Macitentan (CAS: 441798-33-0), an orally active dual endothelin receptor antagonist. Macitentan is a non-chiral sulphadiazine derivative. In vitro studies have shown that it is an oral active antagonist of endothelin ETA and ETB receptor, which can block endothelin-induced vasoconstriction in trachea and aorta. Developed by Swiss company Actelion Pharmaceuticals and first marketed in the United States with FDA approval on October 18, 2013, it is mainly used to treat pulmonary hypertension (PAH) and its product name is Opsumit. Compared with other drugs for pahs, masititan can significantly reduce the morbidity and mortality of pahs patients mainly by slowing the progression of disease.