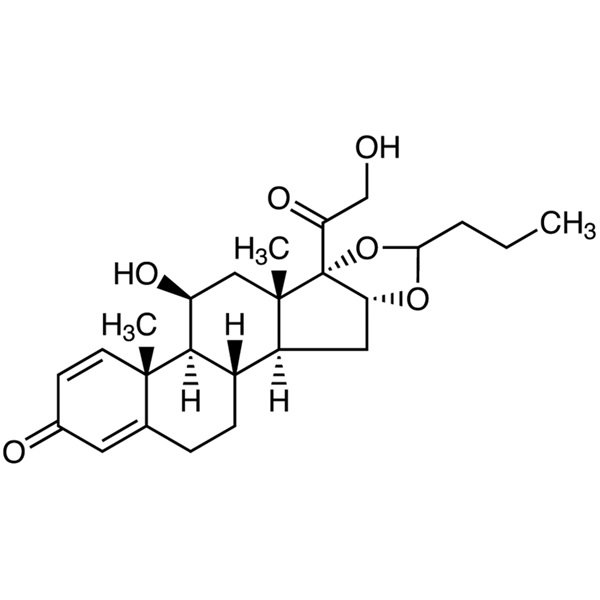
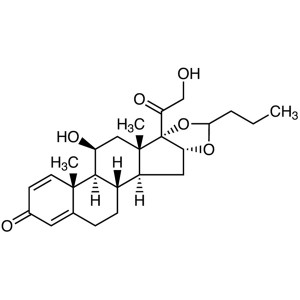
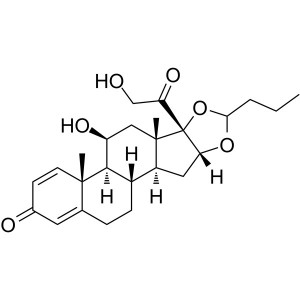
Budesonide CAS 51333-22-3 Assay 98.0~102.0%
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer of Budesonide (CAS: 51333-22-3) with high quality. Ruifu Chemical can provide worldwide delivery, competitive price, excellent service, small and bulk quantities available. Purchase Budesonide, Please contact: alvin@ruifuchem.com
| Chemical Name | Budesonide |
| Synonyms | (+)-16α,17α-Butylidenedioxy-11β,21-Dihydroxy-1,4-Pregnadiene-3,20-Dione; 16,17-Butylidenebis(oxy)-11,21-Dihydroxypregna-1,4-Diene-3,20-Dione; Rhinocort; Pulmicort; Entocort; Symbicort |
| Stock Status | In Stock, Commercial Production |
| CAS Number | 51333-22-3 |
| Molecular Formula | C25H34O6 |
| Molecular Weight | 430.54 g/mol |
| Melting Point | 221.0~232.0℃ |
| Solubility | Insoluble in Water. Soluble in Chloroform, Methanol. Slightly Soluble in Ethanol, Acetone. Very Slightly Soluble in Dichloromethane, Ether |
| COA & MSDS | Available |
| Origin of Product | Shanghai, China |
| Product Categories |
API (Active Pharmaceutical Ingredient) |
| Caution | Not Intended for Human Use. For Research Use Only. |
| Brand | Ruifu Chemical |
| Items | Specifications | Results |
| Appearance | White to Off-White Crystalline Powder | White Crystalline Powder |
| Budesonide Assay | 98.0~102.0% | 98.7% |
| Loss on Drying | <0.30% | 0.19% |
| Any Single Impurity | <0.20% | <0.20% |
| Epimer A | 40.0~51.0% | 47.29% |
| Methanol | <0.10% | 0.005% |
| Infrared Spectrum | Consistent with Structure | Complies |
| 1H NMR Spectrum | Consistent with Structure | Complies |
| Conclusion | The product has been tested and complies with the given specifications | |
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Keep the container tightly closed. Store in a cool, dry (2~8℃) and well-ventilated warehouse away from incompatible substances. Keep away from sunshine; avoid fire and heat sources; avoid moisture.
Shipping: Deliver to worldwide by air, by FedEx / DHL Express. Provide fast and reliable delivery.
Budesonide
C25H34O6 430.53
Pregna-1,4-diene-3,20-dione, 16,17-[1R-butylidenebis(oxy)]-11,21-dihydroxy and pregna-1,4-diene-3,20-dione,16,17-[1S-butylidenebis(oxy)]-11,21-dihydroxy;
(RS)-11,16,17,21-Tetrahydroxypregna-1,4-diene-3,20-dione cyclic 16,17-acetal with butyraldehyde [51372-29-3; 51372-28-2; 51333-22-3].
DEFINITION
Change to read:
Budesonide is a mixture of two epimeric forms, epimer A(C-22S) and epimer B(C-22R). It contains NLT 40.0%(RB 1-Jun-2011) and NMT 51.0% of epimer A, and the sum of both epimers is NLT 98.0% and NMT 102.0% of C25H34O6, calculated on the dried basis.
[Note-Protect all solutions containing budesonide from light.]
IDENTIFICATION
• A. Infrared Absorption <197K>
• B. Ultraviolet Absorption <197U>
Sample solution: 25 µg/mL
Medium: Methanol
Acceptance criteria: Meets the requirements
ASSAY
Change to read:
• Procedure
Buffer: 3.17 mg/mL of monobasic sodium phosphate and 0.23 mg/mL of phosphoric acid. The pH is 3.2 ± 0.1.
Mobile phase: Acetonitrile and Buffer (32:68)
Standard solution: Dissolve a quantity of USP Budesonide RS in acetonitrile, and dilute quantitatively with Buffer to obtain a solution having a concentration of 0.5 mg/mL, keeping the proportion of acetonitrile in this solution to NMT 30%.
Sample solution: Dissolve 25 mg of Budesonide in 15 mL of acetonitrile in a 50-mL volumetric flask, and dilute with Buffer to volume.
Chromatographic system
(See Chromatography <621>, System Suitability.)
Mode: LC
Detector: UV 254 nm
Column: 4.6-mm × 15-cm; 5-µm packing L1
Flow rate: 1.5 mL/min
Injection size: 20 µL
System suitability
Sample: Standard solution
[Note-The relative retention time for epimer A is 1.1, with respect to epimer B.]
Suitability requirements
Resolution: NLT 1.5 between the two budesonide epimer peaks
Column efficiency: NLT 5500 theoretical plates, determined from the budesonide epimer B peak
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of epimer A (C25H34O6) in the portion of Budesonide taken:
Result = [rUA/(rUA + rUB)] × 100
rUA = peak area of epimer A from the Sample solution
rUB = peak area of epimer B from the Sample solution
Calculate the percentage of C25H34O6 in the portion of Budesonide taken:
Result = [(rUA + rUB)/(rSA + rSB)] × (CS/CU) × 100
rUA = peak area of epimer A from the Sample solution
rUB = peak area of epimer B from the Sample solution
rSA = peak area of epimer A from the Standard solution
rSB = peak area of epimer B from the Standard solution
CS = concentration of USP Budesonide RS in the Standard solution (mg/mL)
CU = concentration of Budesonide in the Sample solution (mg/mL)
Acceptance criteria
Epimer A: 40.0%(RB 1-Jun-2011)-51.0% on the dried basis
Both epimers: 98.0%-102.0% on the dried basis
IMPURITIES
• Procedure 1: Limit of 21-Acetate of Budesonide
Buffer: 3.17 mg/mL of monobasic sodium phosphate and 0.23 mg/mL of phosphoric acid. The pH is 3.2 ± 0.1.
Mobile phase: Acetonitrile and Buffer (45:55)
Standard solution: Dissolve a quantity of USP Budesonide RS in acetonitrile, and dilute quantitatively with Buffer to obtain a solution having a concentration of 0.5 mg/mL, keeping the proportion of acetonitrile in this solution to NMT 30%.
Sample solution: Dissolve 25 mg of Budesonide in 15 mL of acetonitrile in a 50-mL volumetric flask, and dilute with Buffer to volume.
Chromatographic system
(See Chromatography <621>, System Suitability.)
Mode: LC
Detector: UV 254 nm
Column: 4.6-mm × 15-cm; 5-µm packing L1
Flow rate: 1.5 mL/min
Injection size: 20 µL
System suitability
Sample: Standard solution
[Note-The relative retention times for the first eluted epimer of the 21-acetate of budesonide, the second eluted epimer of the 21-acetate of budesonide, the first eluted epimer of budesonide (epimer B), and the second eluted epimer of budesonide (epimer A) are 3.1, 3.2, 1.0, and 1.1, respectively. ]
Suitability requirements
Column efficiency: NLT 5500 theoretical plates, determined from the budesonide epimer B peak
Analysis
Sample: Sample solution
Calculate the percentage of the 21-acetate of budesonide in the portion of Budesonide taken:
Result = (rT1/rT2) × 100
rT1 = sum of the peak areas for the two epimers of the 21-acetate of budesonide
rT2 = sum of the peak areas of the two budesonide peaks
Acceptance criteria: NMT 0.10% of the 21-acetate of budesonide is found.
• Procedure 2: Limit of 11-Ketobudesonide
Buffer: 3.17 mg/mL of monobasic sodium phosphate and 0.23 mg/mL of phosphoric acid. The pH is 3.2 ± 0.1.
Mobile phase: Acetonitrile, isopropanol, and Buffer (26:9:65)
Standard solution: Dissolve a quantity of USP Budesonide RS in acetonitrile, and dilute quantitatively with Buffer to obtain a solution having a concentration of 0.5 mg/mL, keeping the proportion of acetonitrile in this solution to NMT 30%.
Sample solution: Dissolve 25 mg of Budesonide in 15 mL of acetonitrile in a 50-mL volumetric flask, and dilute with Buffer to volume.
Chromatographic system
(See Chromatography <621>, System Suitability.)
Mode: LC
Detector: UV 254 nm
Column: 4.6-mm × 15-cm; 3.5-µm packing L1
Column temperature: 50
[Note-Preheat the Mobile phase to 50.]
Flow rate: 1.5 mL/min
Injection size: 20 µL
System suitability
Sample: Standard solution
[Note-The relative retention times for the two epimers of 11-ketobudesonide are 0.73 and 0.78, respectively; the relative retention times for 21-dehydrobudesonide, 14,15-dehydrobudesonide, and the first eluted epimer of budesonide (epimer B) are 0.68, 0.84, and 1.0, respectively.]
Suitability requirements
Column efficiency: NLT 5500 theoretical plates, determined from the budesonide epimer B peak
Analysis
Sample: Sample solution
Calculate the percentage of 11-ketobudesonide in the portion of Budesonide taken:
Result = (rT1/rT2) × 100
rT1 = sum of the peak areas for the two ketobudesonide peaks
rT2 = sum of the peak areas of the two budesonide peaks
Acceptance criteria: NMT 0.2% of 11-ketobudesonide is found.
• Procedure 3
Buffer: 3.17 mg/mL of monobasic sodium phosphate and 0.23 mg/mL of phosphoric acid. The pH is 3.2 ± 0.1.
Mobile phase: Acetonitrile and Buffer (32:68)
Standard solution: Dissolve a quantity of USP Budesonide RS in acetonitrile, and dilute quantitatively with Buffer to obtain a solution having a concentration of 0.5 mg/mL, keeping the proportion of acetonitrile in this solution to NMT 30%.
Sample solution: Dissolve 25 mg of Budesonide in 15 mL of acetonitrile in a 50-mL volumetric flask, and dilute with Buffer to volume.
Chromatographic system
(See Chromatography <621>, System Suitability.)
Mode: LC
Detector: UV 254 nm
Column: 4.6-mm × 15-cm; 5-µm packing L1
Flow rate: 1.5 mL/min
Injection size: 20 µL
System suitability
Sample: Standard solution
Suitability requirements
Column efficiency: NLT 5500 theoretical plates, determined from the budesonide epimer B peak
Analysis
Sample: Sample solution
Calculate the percentage of each impurity in the portion of Budesonide taken:
Result = (rU/rT) × 100
rU = peak area for each impurity
rT = sum of the areas of all of the peaks
Acceptance criteria: See Table 1.
Table 1 Name Relative Retention Time Acceptance Criteria, NMT (%)
16-Hydroxyprednisolonea 0.11 0.2
d-Homobudesonideb 0.36 0.10
21-Dehydrobudesonide (epimers)c 0.61; 0.66 0.07d
14,15-Dehydrobudesonidee 0.86 0.10
Total specified impurities - 0.4f
Any other individual impurity - 0.10
Total unspecified impurities - 0.4
a 11,16,17,21-Tetrahydroxypregna-1,4-diene-3,20-dione.
b 16,17-[(1RS)-Butylidenebis(oxy)]-11-hydroxy-17-(hydroxymethyl)-d-homoandrosta-1,4-diene-3,17a-dione.
c 16,17-[(1RS)-Butylidenebis(oxy)]-11-hydroxy-3,20-dioxopregna-1,4-dien-21-al.
d Limit includes both epimers.
e 16,17-[(1RS)-Butylidenebis(oxy)]-11,21-dihydroxypregna-1,4,14-triene-3,20-dione.
f Total specified impurities includes 11-ketobudesonide obtained in the test for Limit of 11-Ketobudenoside and the impurities listed above.
SPECIFIC TESTS
• Microbial Enumeration Tests <61> and Tests for Specified Microorganisms <62>: The total aerobic microbial count is NMT 103 cfu/g, and the total combined molds and yeast count is NMT 102 cfu/g.
• Loss on Drying <731>: Dry a sample at 105 to constant weight: it loses NMT 0.3% of its weight.
ADDITIONAL REQUIREMENTS
• Packaging and Storage: Preserve in tight, light-resistant containers. Store at controlled room temperature.
• USP Reference Standards <11>
USP Budesonide RS
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc.
Advantages? Superior quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
Hazard Symbols Xn - Harmful
Risk Codes R40 - Limited evidence of a carcinogenic effect
R36/37/38 - Irritating to eyes, respiratory system and skin.
R20/21/22 - Harmful by inhalation, in contact with skin and if swallowed.
Safety Description S22 - Do not breathe dust.
S36 - Wear suitable protective clothing.
S26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
WGK Germany 3
RTECS TU3723000
HS Code 2937 2900.99
Budesonide (CAS: 51333-22-3) (brand name: Pulmicort) is a synthetic corticosteroid medication. Budesonide is indicated for the maintenance and prophylactic treatment of asthma. It is also used for the long-term management of chronic obstructive pulmonary disease (COPD). Budesonide is also useful for the treatment of inflammatory bowel disease including Crohn’s disease, ulcerative colitis and microscopic colitis, and also for treating allergic rhinitis. Budesonide exhibits potent glucocorticoid activity and weak mineralocorticoid activity in vivo. It is effective in inhibiting the activities of multiple cell types and mediators participating in either allergic or non-allergic-mediated inflammation.
Budesonide is a commonly used in the treatment of bronchial asthma clinical drug in China.it can be used for non-hormone-dependent or hormone-dependent asthma, and chronic asthmatic bronchitis. Or it is used for the treatment of seasonal allergic rhinitis and many years occurring allergic rhinitis and it is used for preventing polyps regeneration after the removal of nasal polyps.