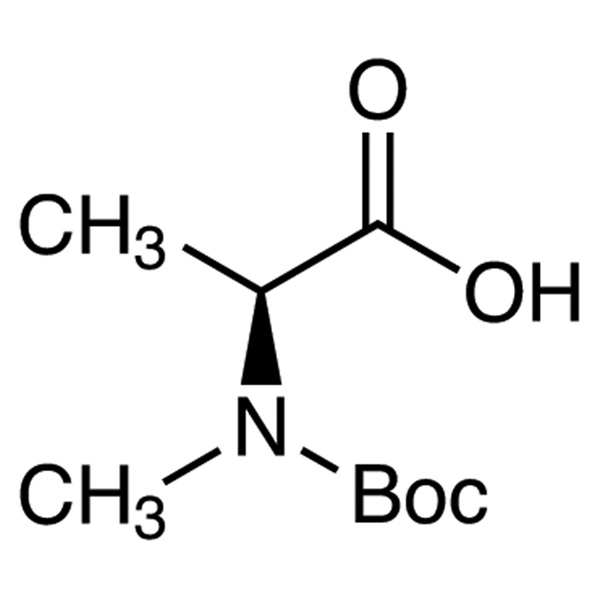
Boc-N-Methyl-L-Alanine (Boc-N-Me-Ala-OH) CAS 16948-16-6 Purity ≥98.0% (HPLC)
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer of Boc-N-Methyl-L-Alanine (Boc-N-Me-Ala-OH) (CAS: 16948-16-6) with high quality. Boc-N-Me-Ala-OH is widely used in peptide synthesis, biochemistry, and drug discovery and development. Ruifu Chemical has been supplying amino acid derivatives more than 15 years.
Ruifu Chemical can provide worldwide delivery, competitive price, excellent service.
Purchase Boc-N-Methyl-L-Alanine or other products, please contact us by e-mail: alvin@ruifuchem.com
| Chemical Name | Boc-N-Methyl-L-Alanine |
| Synonyms | Boc-N-Me-Ala-OH; Boc-N-Me-L-Ala-OH; Boc-L-N-Me-Ala-OH; Boc-N-Methyl-L-Ala-OH; N-Boc-N-Methyl-L-Alanine; N-(tert-Butoxycarbonyl)-N-Methyl-L-Alanine; N-tert-Butoxycarbonyl-N-Methylalanine; Boc-L-MeAla-OH |
| Stock Status | In Stock, Commercial Production |
| CAS Number | 16948-16-6 |
| Molecular Formula | C9H17NO4 |
| Molecular Weight | 203.24 g/mol |
| Melting Point | 85.0-91.0℃ |
| Density | 1.111±0.06 g/cm3 |
| Store Under Inert Gas | Store Under Inert Gas |
| Sensitive | Air Sensitive |
| Storage Temperature | Store Long-Term at 2-8℃ |
| COA & MSDS | Available |
| Origin of Product | Shanghai, China |
| Product Categories |
N-Methyl Amino Acids |
| Brand | Ruifu Chemical |
| Items | Specifications | Results |
| Appearance | White to Off-White Powder | White Powder |
| Specific Rotation [α]20/D | -29.0° to -31.0° (C=1 in EtOH) | -29.23° |
| Melting Point | 85.0-91.0℃ | 89.0-91.0℃ |
| Loss on Drying | ≤0.50% | 0.19% |
| Purity / Analysis Method | ≥98.0% (HPLC) | 99.73% |
| Identification (MS) | In Accordance with the Standard | Conforms |
| Identification (NMR) | In Accordance with the Standard | Conforms |
| Conclusion | The product has been tested and complies with the given specifications | |
Package: Fluorinated bottle, Aluminium foil bag, 25kg/cardboard drum, or according to customer's requirement.
Storage Condition: Keep the container tightly closed. Store in a cool, dry (2~8℃) and well-ventilated warehouse away from incompatible substances. Keep away from sunshine; avoid fire and heat sources; avoid moisture.
Shipping: Deliver to worldwide by air, by FedEx / DHL Express. Provide fast and reliable delivery.
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc.
Advantages? Superior quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
2 Test Procedures
2.1 Identity by HPLC
Note Perform this test only if it is listed in the release specifications
Principle Comparison of the retention times of the sample and the comparison substances
Method See Related Substances by HPLC (area normalization)
Chromatograms See Related Substances by HPLC (area normalization)
Evaluation Compare the retention times of the main peak in the chromatograms of the sample and the comparison substance. Calculate the difference between the retention times in min.
Diff (min) = | RTss - RTcs |
Evaluation/assessment Criteria are indicated in the specifications.
If a retention time requirement is included in the chromatographic suitability evaluation, the same acceptance criteria will be utilized in the evaluation of the retention time for identity.
RTss Retention time of the main peak in the sample solution
RTcs Retention time of the main peak in the comparison solution
2.2 Related Substances by HPLC (area normalization)
Principle RP HPLC with UV detection
Assessment in area %
Reagents
Water e.g. Milli-Q-equipment
Acetonitrile Gradient grade, e.g. Sigma-Aldrich 34851
Ortho-phosphoric acid 85% e.g. Fluka 79606
Solvent Acetonitrile / water, 50:50 (v/v)
Mix 500 mL acetonitrile and 500 mL water
Comparison substance(s) Current comparison substance batches of:
• N-tert-Butoxycarbonyl-N-methyl-L-alanine
• N-tert-Butoxycarbonyl-L-alanine
Equipment
Apparatus e.g. Agilent 1200 HPLC
Column Zorbax SB-C18
Length 150 mm, internal diameter 3.0 mm or equivalent column, particle size: 3.5 µm
Note: The dimensions of the column (max. changes: column length ± 70%; inner diameter ± 25%) and the particle size (max. changes - 50%) may be changed as long as the requirements of the system suitability test are met (values for allowed changes are taken from Ph Eur 2.2.46).
Test replicates Each sample, singularly
Chromatographic conditions
Mobile phase A 0.1% H 3 PO 4 in water
Pipette 1 mL of 85% ortho-phosphoric acid in 1000 mL of water
Mobile Phase B Acetonitrile
Gradient Time [min.] Phase B [%]
0 10
10 45
15 95
20 95 End of acquisition
20.1 10
30 10 Next injection (Run time)
Flow rate 0.5 mL/min
Detection UV 210 nm
Temperature 30 °C
Injection volume 5 µL
SST parameters
Specificity Solution for specificity
Reporting limit (= LOQ) 0.10%
SST requirements
Specificity The specificity must be equivalent to that of the comparison chromatograms (see Fig. 5-2)
Reporting limit (= LOQ) The signal-to-noise ratio must be equal or greater than 10 or s rel ≤ 15% (peak area of N-tert-butoxycarbonyl-N-methyl-L-alanine in LOQ solution, n = 3)
Note: If more than 5 sample solutions are injected or the runtime of the sequence after the SST is longer than 5 h, the SST has to be repeated at the end of the sequence.
Test Procedure
Order of injections Example 1: Blank, LOQ (1x or 3x), specificity solution, comparison solution*, sample solution (up to 5)
Example 2: Blank, LOQ (1x or 3x), specificity solution, comparison solution*, sample solution (more than 5), LOQ (1x or 3x), specificity
*needed in combination with “5.2 Identity by HPLC” only
Sample solution Weigh 120-130 mg of the sample accurately to 0.1 mg into a 25 mL volumetric flask, dissolve in and dilute to volume with solvent.
Comparison solution (for Identity by HPLC only)
Weigh 48-52 mg of the N-tert-butoxycarbonyl-N-methyl-L-alanine comparison substance accurately to 0.1 mg into a 10 mL volumetric flask, dissolve in and dilute to volume with solvent.
LOQ solution Dilute 25 µL of sample solution to a volume of 25 mL with solvent.
Specificity solution Weigh 8-10 mg of N-tert-butoxycarbonyl-L-alanine comparison substance into a 10 mL volumetric flask, dissolve in and dilute to volume with sample solution.
Evaluation By peak area ratio (area %)
See sample solution chromatogram (see Fig. 2-1)
Figure 2-1 Overlay chromatograms of a blank (I), LOQ solution (II), a sample solution (III) and the specificity solution (IV)
2.3 Enantiomeric purity by chiral HPLC (area normalization)
Principle Chiral RP HPLC with UV detection
Assessment in area %
Reagents
Water e.g. Milli-Q-equipment
Acetonitrile Gradient grade, e.g. Sigma-Aldrich 34851
Ortho-phosphoric acid 85% e.g. Fluka 79606
Solvent Acetonitrile / water, 50:50 (v/v)
Mix 500 mL acetonitrile and 500 mL water
Comparison substance(s) Current comparison substance batches of:
• N-tert-Butoxycarbonyl-N-methyl-D-alanine
• N-tert-Butoxycarbonyl-N-methyl-L-alanine
Equipment
Apparatus e.g. Agilent 1200 HPLC
Column Daicel chiralcel OJ-RH
Length 150 mm, internal diameter 4.6 mm or equivalent column, particle size: 5 µm
Note: The dimensions of the column (max. changes: column length ± 70%; inner diameter ± 25%) and the particle size (max. changes − 50%) may be changed as long as the requirements of the system suitability test are met (values for allowed changes are taken from Ph Eur 2.2.46).
Test replicates Each sample, singularly
Chromatographic conditions
Mobile phase A 0.1% H 3 PO 4 in water
Pipette 1 mL of 85% ortho-phosphoric acid in 1000 mL of water
Mobile Phase B Acetonitrile
Isocratic separation B = 20 %
Run time 20 min (=time of acquisition)
Flow rate 0.5 mL/min
Detection UV 210 nm
Temperature 30℃
Injection volume 10 µL
SST parameters
Specificity Solution for specificity
Reporting limit (= LOQ) 0.10%
SST requirements
Specificity The specificity must be equivalent to that of the comparison
chromatograms (see Fig. 5-3)
Reporting limit (= LOQ) The signal-to-noise ratio must be equal or greater than 10 or s rel ≤ 15% (peak area of N-tert-butoxycarbonyl-N-methyl-L-alanine in LOQ solution, n = 3)
Note: If more than 5 sample solutions are injected or the runtimem of the sequence after the SST is longer than 5 h, the SST has to be repeated at the end of the sequence.
Test Procedure
Order of injections Example 1: Blank, LOQ (1x or 3x), specificity solution, sample solution (up to 5)
Example 2: Blank, LOQ (1x or 3x), specificity solution, sample solution (more than 5), LOQ (1x or 3x), specificity
Sample solution Weigh 120-130 mg of the sample accurately to 0.1 mg into a 25 mL volumetric flask, dissolve in and dilute to volume with solvent.
LOQ solution Dilute 25 µL of sample solution to a volume of 25 mL with solvent.
Evaluation By peak area ratio (area %)
Figure 2-1 Chromatograms of the specificity solution
| Safety Description | 24/25 - Avoid contact with skin and eyes. |
| WGK Germany | 3 |
| HS Code | 2922491990 |
| Hazard Class | IRRITANT |
Boc-N-Methyl-L-Alanine (Boc-N-Me-Ala-OH) (CAS: 16948-16-6) is an amino acid derivative composed of a methyl group on the alpha carbon atom of alanine, with a tert-butoxycarbonyl (Boc) group attached to the N-terminus of the molecule. The Boc group provides protection to the amino group during peptide synthesis, allowing for selective deprotection of the desired amino acid residues. The compound was first synthesized by R. B. Merrifield in 1963, and since then, it has been extensively used in peptide synthesis, which is an essential tool in the development of drugs, vaccines, and other bioactive molecules.
Boc-N-Methyl-L-Alanine (Boc-N-Me-Ala-OH) (CAS: 16948-16-6) is an amino acid derivative commonly used in peptide synthesis, biochemistry, and drug discovery. Boc-N-Methyl-L-Alanine is a valuable synthetic tool for the production of peptides, proteins, and other bioactive molecules. Boc-N-Methyl-L-Alanine is used in a variety of applications such as drug discovery, drug development, and biochemistry.
Boc-N-Methyl-L-Alanine has numerous applications in scientific research, particularly in the areas of peptide synthesis and drug design. Boc-N-Methyl-L-Alanine can be used as a protected amino acid in the solid-phase peptide synthesis, facilitating the assembly of peptide chains with high yield and purity.
In the pharmaceutical industry, Boc-N-Methyl-L-Alanine can be utilized in the development of novel drugs and vaccines, by providing a key building block for the assembly of complex molecules.