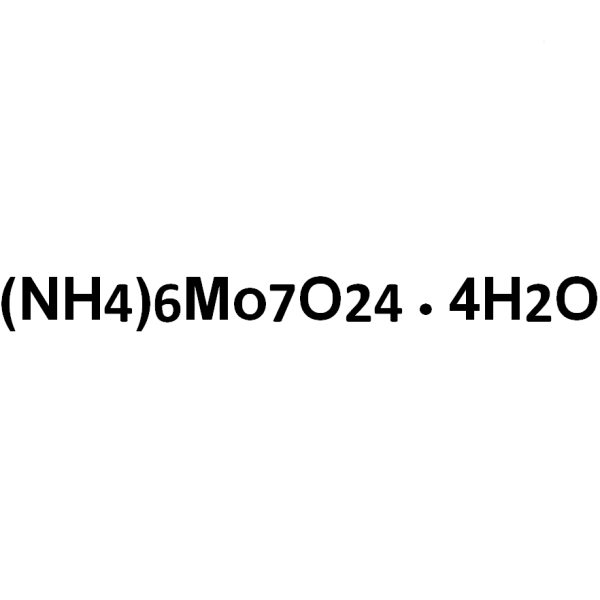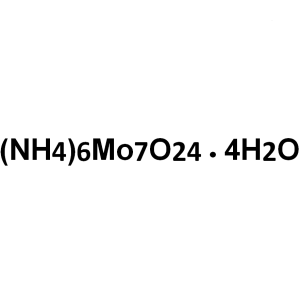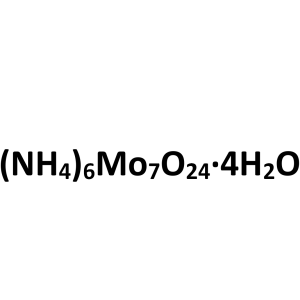Ammonium Molybdate Tetrahydrate CAS 12054-85-2 Purity >99.0% Molybdenum (Mo) >54.0%
Manufacturer Supply With High Quality, Commercial Production
Chemical Name: Ammonium Molybdate Tetrahydrate CAS: 12054-85-2
| Chemical Name | Ammonium Molybdate Tetrahydrate |
| Synonyms | AMT; Ammonium Molybdate 4-Hydrate; Ammonium Molybdate(Vi) Tetrahydrate; Molybdic Acid Ammonium Tetrahydrate; Ammonium Heptamolybdate Tetrahydrate |
| CAS Number | 12054-85-2 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | Mo7O24 .6(NH4).4(H2O) |
| Molecular Weight | 1235.85 |
| Melting Point | 190℃ |
| Density | 2.498 g/ml at 25℃ (lit.) |
| Water Solubility | 400 g/L (20℃) |
| Solubility | Soluble in Water, Insoluble in Alcohol |
| Stability | Stable. Incompatible with Strong Acids, Strong Oxidizing Agents. |
| COA & MSDS | Available |
| Place of Origin | Shanghai, China |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | White Crystalline |
| Assay | 99.44% |
| Molybdenum (Mo) | 54.03% |
| Silicon (Si) | 0.0006% |
| Lead (Pb) | 0.0005% |
| lron (Fe) | 0.0005% |
| Sulfate (SO4) | 0.015% |
| Aluminum (Al) | 0.0005% |
| Chloride (Cl) | 0.002% |
| Water Insoluble Mater | 0.01% |
| Copper (Cu) | 0.0003% |
| Manganese (Mn) | 0.0005% |
| Phosphorus (P) | 0.0005% |
| Bismuth (Bi) | 0.0006% |
| Stannum (Sn) | 0.0006% |
| Cadmium (Cd) | 0.0005% |
| Nickel (Ni) | 0.0004% |
| Arsenic (as As) | 0.0005% |
| Test Standard | Enterprise Standard |
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum/Bag, or according to customer's requirement.
Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture.

Risk Codes R36/37/38 - Irritating to eyes, respiratory system and skin.
R22 - Harmful if swallowed
R20/21/22 - Harmful by inhalation, in contact with skin and if swallowed.
Safety Description
S26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S37/39 - Wear suitable gloves and eye/face protection
S36/37 - Wear suitable protective clothing and gloves.
S36 - Wear suitable protective clothing.
UN IDs 3288
WGK Germany 1
RTECS QA5076000
TSCA Yes
HS Code 2841701000
Ammonium Molybdate Tetrahydrate (CAS: 12054-85-2) has many applications, e.g. as an analytic reagent for measuring content of phosphates, silicates, arsenates and lead; for the production of molybdenum metal and ceramics; for the production of dehydrogenation and desulphurization catalysts; for the fixing of metals; for electroplating; supplement in the crop fertilizer; as a negative stain in biological electron microscopy; as an analgesic in medical fields.
Ammonium Molybdate
(NH4)6Mo7O24·4H2O 1235.86
Molybdate (Mo7O246-), hexaammonium, tetrahydrate;
Hexaammonium molybdate tetrahydrate [12054-85-2].
DEFINITION
Ammonium Molybdate contains NLT 99.3% and NMT 101.8% of (NH4)6Mo7O24·4H2O.
IDENTIFICATION
• Procedure
Sample: 0.6 g
Analysis: Dissolve the Sample in 1.4 mL of water and 1.45 mL of ammonium hydroxide. Cool this mixture, and add slowly, with mixing, 7.2 mL of a well-cooled mixture of 3.2 mL of nitric acid and 4 mL of water. Allow to stand for 24–48 h, and pass through a sintered-glass filter. To 5 mL of the filtrate add 2 mL of dibasic sodium phosphate TS.
Acceptance criteria: A yellow precipitate is formed, and it is soluble in an excess of 6 N ammonium hydroxide.
ASSAY
• Procedure
Sample solution: Dissolve 0.7 g of Ammonium Molybdate in 100 mL water. Adjust with dilute nitric acid to a pH of 4.0. Add saturated hexamethylenetetramine solution to achieve a pH of 5–6.
Analysis: Heat the Sample solution to 60, and add 0.2 mL of 0.1% 4-[2-pyridylazo]resorcinol solution in alcohol. Titrate with 0.1 M lead nitrate VS from the yellow color to the first permanent pink endpoint. Carry out a blank titration. Each mL of 0.1 M lead nitrate is equivalent to 17.66 mg of (NH4)6Mo7O24·4H2O.
Acceptance criteria: 99.3%-101.8%
IMPURITIES
Inorganic Impurities
• Arsenate, Phosphate, and Silicate
Sample solution: Dissolve 2.5 g of the analyte in 70 mL of water in a container other than glass.
Control solution: Dissolve 0.5 g of the analyte in 70 mL of water in a container other than glass, and add an amount of sodium silicate solution equivalent to 0.02 mg of silica (SiO2).
Analysis
Samples: Sample solution and Control solution
Adjust with 1.2 N hydrochloric acid to a pH of between 3 and 4, transfer to a glass container, add 2 mL of bromine TS, and adjust with 1.2 N hydrochloric acid to a pH of 1.8 ± 0.1. Heat almost to boiling, and cool to room temperature. Dilute with water to 90 mL, add 10 mL of hydrochloric acid, and transfer to a separator. Add 1 mL of butyl alcohol and 30 mL of 4-methyl-2-pentanone, shake vigorously, and allow the phases to separate. Discard the aqueous phase, and wash the ketone phase with three successive 10-mL portions of 1.2 N hydrochloric acid, discarding the washings. To the washed ketone phase add 10 mL of 1.2 N hydrochloric acid to which has just been added 0.2 mL of a freshly prepared solution (1 in 50) of stannous chloride in hydrochloric acid.
Acceptance criteria: Any blue color in the Sample solution does not exceed that in the Control solution (NMT 10 ppm).
• Chloride and Sulfate, Chloride 221: A 0.5-g portion shows no more chloride than 0.30 mL of 0.001 N hydrochloric acid (NMT 20 ppm).
• Chloride and Sulfate, Sulfate 221: A 0.25-g portion shows no more sulfate than corresponds to 1.0 mL of 0.001 N sulfuric acid (NMT 200 ppm).
• Heavy Metals <231>
Sample stock solution: Dissolve 2.0 g of Ammonium Molybdate in 20 mL of water, add 10 mL of 2.5 N sodium hydroxide and 2 mL of ammonium hydroxide, and dilute with water to 40 mL.
Control solution: To 10 mL of Sample stock solution add 1.0 mL of Standard Lead Solution, prepared as directed under Heavy Metals 231, and dilute with water to 40 mL.
Sample solution: Dilute the remaining 30-mL portion of the Sample stock solution with water to 40 mL.
Analysis: To both the Sample solution and the Control solution add 1.2 mL of thioacetamide–glycerin base TS and 2 mL of pH 3.5 Acetate Buffer, and allow to stand for 5 min.
Acceptance criteria: Any color in the Sample solution does not exceed that in the Control solution (NMT 10 ppm).
• Insoluble Substances
Sample: 20 g
Analysis: Dissolve the Sample in 200 mL of water in a beaker, heat to boiling, cover, and heat on a steam bath for 1 h. Pass the hot solution through a tared filtering crucible, wash the insoluble residue with hot water, and dry at 105 for 2 h.
Acceptance criteria: The weight of the residue is NMT 1 mg (0.005%).
• Nitrate
Sample: 1 g
Analysis: Dissolve the Sample in 10 mL of water containing 5 mg of sodium chloride, and add 0.10 mL of a solution (1 in 1000) of indigo carmine in 3.6 N sulfuric acid.
Acceptance criteria: The blue color is not completely discharged in 5 min.
• Magnesium and Alkali Salts
Sample: 5.0 g
Analysis: Dissolve the Sample in 50 mL of water, and filter. To the filtrate add 0.5 g of sodium carbonate and 25 mL of 2.5 N sodium hydroxide. Boil the solution gently for 5 min, cool, and pass through an ignited and tared filter. Wash the filter with 1 N ammonium hydroxide. Ignite the filter at 800 ± 25 for 30 min.
Acceptance criteria: The weight of the residue does not exceed 1 mg (NMT 0.02%).
• Phosphate
Standard solution: Dissolve 143.3 mg of dried monobasic potassium phosphate in water to make 1000 mL, and then dilute 1.0 mL of this solution with 3 N ammonium hydroxide to 100 mL.
Sample solution: Dissolve 20 g of the analyte in 100 mL of 3 N ammonium hydroxide.
Analysis
Samples: Standard solution and Sample solution
Add 3.5 mL of ferric nitrate solution (1 in 10), and allow to stand for 15 min. Warm gently to coagulate the precipitate, and filter. Wash the filter several times with 1.5 N ammonium hydroxide, then wash the filter with 60 mL of warm 4 N nitric acid to dissolve the residue on the filter, collecting the filtrate in a glass-stoppered, 250-mL conical flask. Add 13 mL of ammonium hydroxide, warm to 40, add 50 mL of ammonium molybdate TS, shake for 5 min, and allow to stand at 40 for 2 h.
Acceptance criteria: Any yellow precipitate formed from the Sample solution does not exceed that from the Standard solution (5 ppm).
ADDITIONAL REQUIREMENTS
• Packaging and Storage: Preserve in tight containers.