Allylboronic Acid Pinacol Ester CAS 72824-04-5 Purity >98.0% (GC) Factory High Quality
Manufacturer Supply With High Quality, Commercial Production
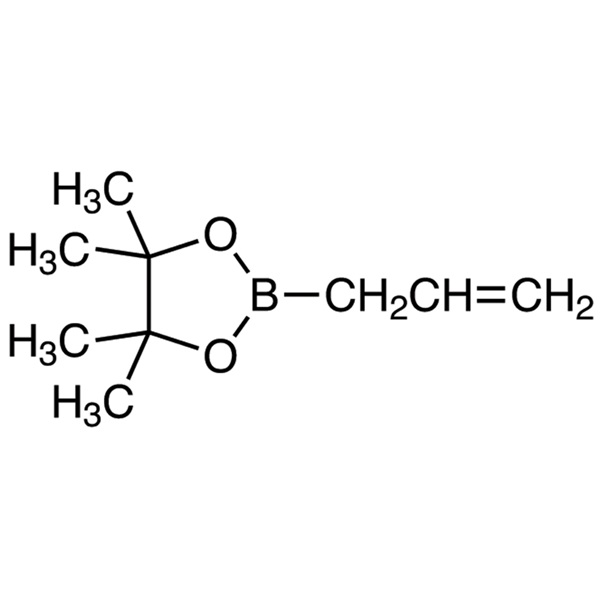
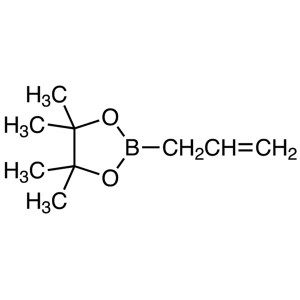
Chemical Name: Allylboronic Acid Pinacol Ester CAS: 72824-04-5
| Chemical Name | Allylboronic Acid Pinacol Ester (Stabilized With Phenothiazine) |
| Synonyms | 2-Allyl-4,4,5,5-Tetramethyl-1,3,2-Dioxaborolane; 2-(Prop-2-en-1-yl)-4,4,5,5-Tetramethyl-1,3,2-Dioxaborolane (Stabilized With Phenothiazine) |
| CAS Number | 72824-04-5 |
| CAT Number | RF-PI1390 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C9H17BO2 |
| Molecular Weight | 168.04 |
| Boiling Point | 50.0~53.0℃/5 mmHg (lit.) |
| Density | 0.896 g/ml at 25℃ (lit.) |
| Refractive Index (N20/D) | 1.425~1.427 |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | Colorless to Pale Yellow Liquid |
| Purity / Analysis Method | >98.0% (GC) |
| Stabilized With Phenothiazine | <2.00% |
| Total Impurities | <2.00% |
| Test Standard | Enterprise Standard |
| Usage | Pharmaceutical Intermediates |
Package: Bottle, 25kg/Barrel, or according to customer's requirement.
Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture.


Allylboronic Acid Pinacol Ester (CAS: 72824-04-5) can act as reagent used for Palladium-catalyzed Suzuki-Miyaura cross-coupling reactions and olefin metathesis; Intermolecular radical additions; Allylboration of aldehydes catalyzed by chiral spirobiindane diol (SPINOL) based phosphoric acids and Cobalt-catalyzed regioselective hydrovinylation of dienes with alkenes; Nucleic acid-templated energy transfer leading to a photorelease reaction and stereoselective indium-catalyzed Hosomi-Sakurai reactions. Allylboronic Acid Pinacol Ester reacts with carboxylic acids, in the presence of tri-n-butyltin hydride, to give homoallylic alcohols in good yield. Homoallylic alcohols can also be formed by allylboration of aldehydes.