6-O-Methylguanine CAS 20535-83-5 Nelzarabine Intermediate Factory
Supply With High Purity, Commercial Production
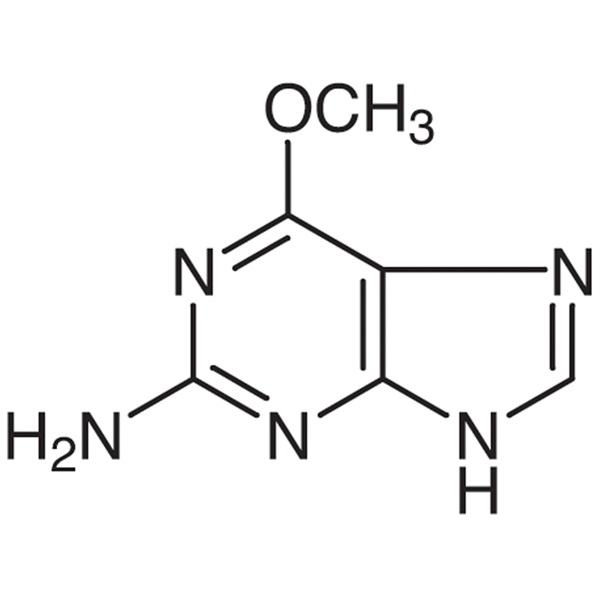
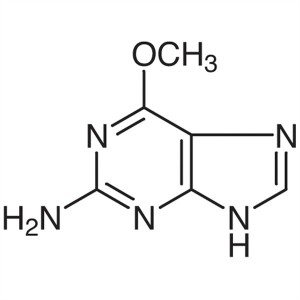
Chemical Name: 6-O-Methylguanine
CAS: 20535-83-5
| Chemical Name | 6-O-Methylguanine |
| Synonyms | 2-Amino-6-Methoxypurine |
| CAS Number | 20535-83-5 |
| CAT Number | RF-PI509 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C6H7N5O |
| Molecular Weight | 165.16 |
| Melting Point | >300℃ (lit.) |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | Off-White Crystalline Powder |
| Assay / Analysis Method | ≥98.5% (HPLC) |
| Loss on Drying | ≤0.50% |
| Residue on Ignition | ≤0.50% |
| Total Impurities | ≤1.5% |
| Test Standard | Enterprise Standard |
| Usage | Intermediate of Nelzarabine (CAS: 121032-29-9), T-ALL & T-LBL |
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture.


6-O-Methylguanine (CAS: 20535-83-5) can be used as an intermediate in the synthesis of Nelzarabine (CAS: 121032-29-9). Nelarabine is a prodrug for deoxyguanosine analogue-9-beta-D-arabinofuranosyl guanine (ara-G). Nelarabine demethylates in the role of adenosine deaminase (ADA) and turns into ara-G. Nelarabine was first successfully developed by GlaxoSmithKline. On October 28, 2005 under the approval of the US Food and Drug Administration, it became a new drug for curing T-cell acute lymphoblastic leukemia (T-ALL) and T-Cells lymphoblasticlymphoma (T-LBL) which are resistant to at least two types of chemotherapy regimens or relapsed after initial treatment. This new drug officially listed in the United States in 2006.