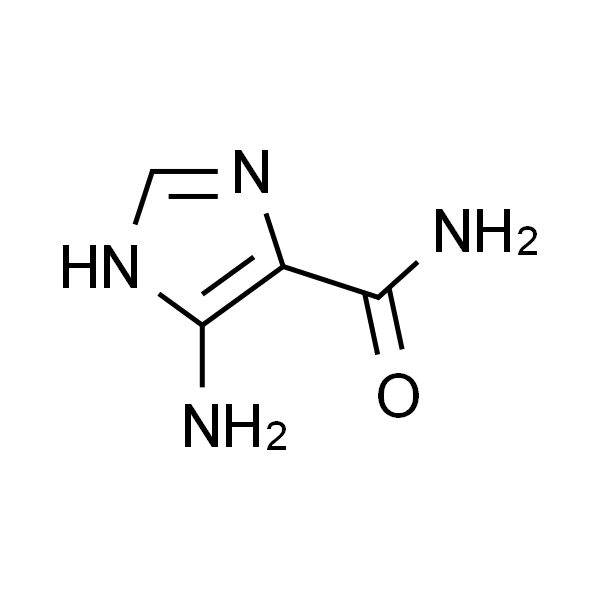
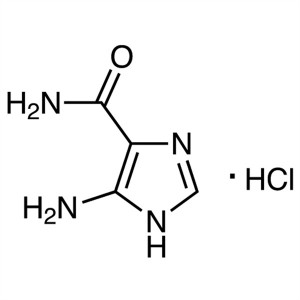
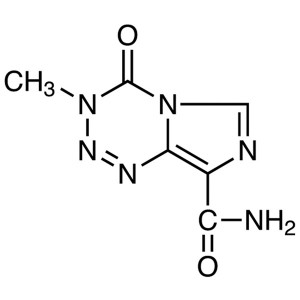
4(5)-Amino-5(4)-Imidazolecarboxamide CAS 360-97-4 Assay ≥99.5% (HPLC) Temozolomide Intermediate Factory
Manufacturer with High Purity and Commercial Production
Supply Temozolomide and Related Intermediates:
Temozolomide CAS: 85622-93-1
4(5)-Amino-5(4)-Imidazolecarboxamide CAS: 360-97-4
5(4)-Amino-4(5)-Imidazolecarboxamide Hydrochloride CAS: 72-40-2
| Chemical Name | 4(5)-Amino-5(4)-Imidazolecarboxamide |
| Synonyms | 4(5)-Aminoimidazole-5(4)-Carboxamide; 5-Amino-3H-Imidazole-4-Carboxamide; AICA |
| CAS Number | 360-97-4 |
| CAT Number | RF-PI936 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C4H6N4O |
| Molecular Weight | 126.12 |
| Solubility | Soluble in DMSO, Methanol, Water |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | White to Similar White Crystal Powder |
| Assay / Analysis Method | ≥99.5% (HPLC) |
| Melting Point | 169.0~173.0℃ |
| Loss on Drying | ≤12.5% |
| Heavy Metals (as Pb) | ≤20ppm |
| Solubility | Clear (5% in Water) |
| Individual Impurity | ≤0.20% |
| Total Impurities | ≤0.50% |
| Residual Solvents | |
| Xylene | ≤0.20% |
| Ethanol | ≤0.50% |
| Test Standard | Enterprise Standard |
| Usage | Intermediate of Temozolomide (CAS: 85622-93-1) |
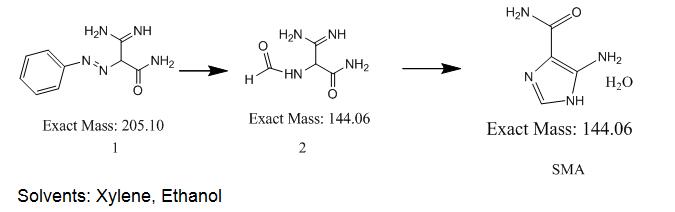
Synthetic Route (CAS 360-97-4)
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture.


4(5)-Amino-5(4)-Imidazolecarboxamide (CAS: 360-97-4) is metabolite of Temozolomide (CAS: 85622-93-1), used as an intermediate of Temozolomide. Temozolomide is the first effective orally-taken imidazole and tetrazine-class anticancer drug which belongs to the second generation of an alkylating agent with antitumor activity without liver metabolic activation after oral administration. Temozolomide was launched for the first time in the UK for the treatment of patients with glioblastoma multiforme showing recurrence or progression after standard therapy.