4-Chlorophenylboronic Acid CAS 1679-18-1 Purity >99.5% (HPLC) Factory High Quality
Manufacturer Supply With High Quality, Commercial Production
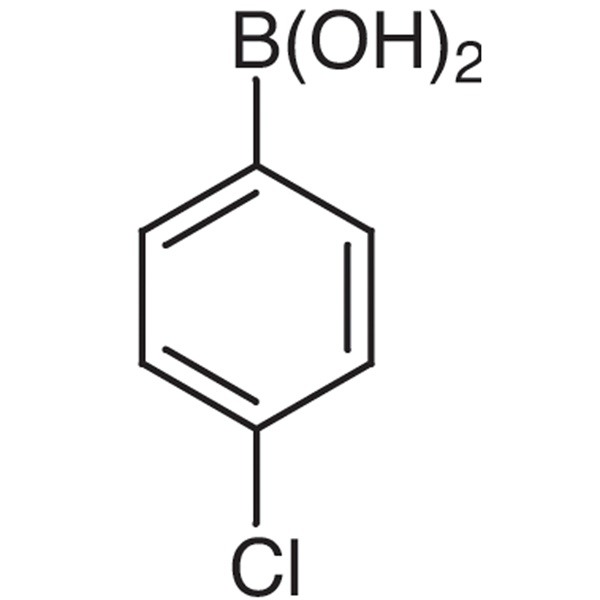
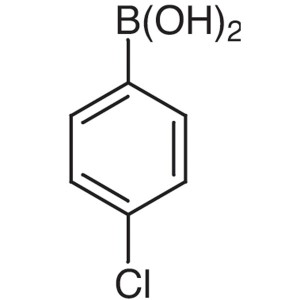
Chemical Name: 4-Chlorophenylboronic Acid CAS: 1679-18-1
| Chemical Name | 4-Chlorophenylboronic Acid |
| Synonyms | 4-Chlorobenzeneboronic Acid; p-Chlorophenylboronic Acid |
| CAS Number | 1679-18-1 |
| CAT Number | RF-PI1315 |
| Stock Status | In Stock, Production Scale Up to 25 Tons/Month |
| Molecular Formula | C6H6BClO2 |
| Molecular Weight | 156.37 |
| Solubility | Soluble in Methanol; Slightly Soluble in Water |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | White to Off-White Powder |
| Purity / Analysis Method | >99.5% (HPLC) |
| Melting Point | 284.0~289.0℃ |
| Moisture (K.F) | <0.50% |
| Residue on Ignition | <0.20% |
| Single Impurity | <0.50% |
| Total Impurities | <0.50% |
| Heavy Metals (as Pb) | <20ppm |
| Test Standard | Enterprise Standard |
| Usage | Pharmaceutical Intermediates; OLED Intermediates |
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture.


4-Chlorophenylboronic Acid (CAS: 1679-18-1), can be used as pharmaceutical and material intermediates. It is also an important intermediate for the OLED production, widely used in electronic materials. 4-Chlorophenylboronic Acid can be used as a reactant in: Suzuki-Miyaura Cross Coupling Reaction; Palladium-catalyzed direct arylation; Cyclopalladation; Tandem-type Pd(II)-catalyzed oxidative Heck reaction and intramolecular C-H amidation; Copper-mediated ligandless aerobic fluoroalkylation; Pd-catalyzed arylative cyclization. Ruthenium catalyzed direct arylation; Ligand-free copper-catalyzed coupling reactions; Regioselective arylation and alkynylation by Suzuki-Miyaura and Sonogashira cross-coupling reactions. It can also be used to prepare: Substituted diarylmethylidenefluorenes via Suzuki coupling reaction; Baclofen lactam by Suzuki coupling of a pyrrolinyl tosylate, followed by hydrogenation reaction; Palladium(II) thiocarboxamide complexes as Suzuki coupling catalysts.