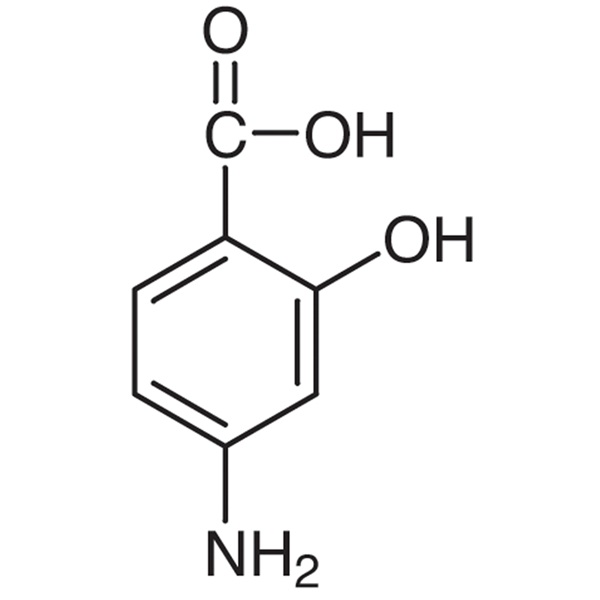
4-Aminosalicylic Acid CAS 65-49-6 Assay 98.5%~101.5% USP35 Standard Factory
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer and supplier of 4-Aminosalicylic Acid (CAS: 65-49-6) with high quality. Ruifu Chemical can provide worldwide delivery, small and bulk quantities available. If you are interested in this product, please send detailed information includes CAS number, product name, quantity to us. Please contact: alvin@ruifuchem.com
| Chemical Name | 4-Aminosalicylic Acid |
| Synonyms | 4-Amino-2-Hydroxybenzoic Acid; 4-Amino-Salicylic Acid; 4-ASA; PAS; Para-Aminosalicylic Acid; p-Aminosalicylic Acid; Aminosalicylic Acid; Amino-PAS; 4-Aminosalicylate |
| CAS Number | 65-49-6 |
| Stock Status | In Stock, Production Capacity 30 Tons per Month |
| Molecular Formula | C7H7NO3 |
| Molecular Weight | 153.14 |
| Melting Point | 135.0~145.0℃(lit.) |
| Sensitive | Hygroscopic. Light Sensitive, Air Sensitive |
| Solubility in Methanol | Almost Transparency |
| COA & MSDS | Available |
| Origin of Product | Shanghai, China |
| Product Categories | Pharmaceutical Intermediates |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | Off-White or Slightly Yellow Powder |
| Identification | Onset Positive Reaction |
| Clarity and Color of Solution | Complies |
| Purity / Analysis Method | >99.0% (HPLC) |
| Purity / Analysis Method | 98.5~101.5% (Titration by NaOH) |
| Melting Point | 135.0~145.0℃ |
| Loss on Drying | <0.50% |
| Residue on Ignition | <0.20% |
| Chloride (Cl) | <0.042% |
| Heavy Metals | <30ppm |
| m-Aminophenol Content | <0.25% (HPLC) |
| Hydrogen Sulfide, Sulfur Dioxide and Amyl Alcohol | Complies |
| Total Impurities | <1.00% |
| pH | 3.0~3.7 |
| Assay / Analysis Method | 98.5~101.5% |
| Infrared Spectrum | Conforms to Structure |
| Test Standard | This Product by Inspection Accords With the Standard USP35 |
Aminosalicylic Acid
C7H7NO3 153.14
Benzoic acid, 4-amino-2-hydroxy-.
4-Aminosalicylic acid [65-49-6].
» Aminosalicylic Acid contains not less than 98.5 percent and not more than 100.5 percent of C7H7NO3, calculated on the anhydrous basis.
[Caution-Under no circumstances use a solution prepared from Aminosalicylic Acid if its color is darker than that of a freshly prepared solution.]
Packaging and storage- Preserve in tight, light-resistant containers, at a temperature not exceeding 30.
USP Reference standards <11>-
USP Aminosalicylic Acid RS
USP m-Aminophenol RS
Clarity and color of solution- One g dissolves in 10 mL of sodium bicarbonate solution (1 in 15) to form a clear solution that has not more than a faint yellow color. One g dissolves in a freshly prepared mixture of 5 mL of nitric acid and 45 mL of water to form a clear solution that has not more than a slight color.
Identification-
A: Dissolve 0.25 g in 3 mL of 1 N sodium hydroxide, transfer to a 500-mL volumetric flask, dilute with water to volume, and mix. Transfer a 5-mL aliquot to a 250-mL volumetric flask containing 12.5 mL of pH 7 phosphate buffer (see Buffer Solutions in the section Reagents, Indicators, and Solutions), dilute with water to volume, and mix. This solution, when compared in a suitable spectrophotometer against a blank of the same buffer in the same concentration, exhibits absorbance maxima at 265 ± 2 and 299 ± 2 nm, and the ratio A265/A299 is between 1.50 and 1.56.
B: Place about 1 g in a small, round-bottom flask, and add 10 mL of acetic anhydride. Heat the flask on a steam bath for 30 minutes, add 40 mL of water, mix, filter, cool, and allow to stand until the diacetyl derivative has crystallized. Collect the precipitate on a filter, wash well with water, and dry at 105 for 1 hour: the diacetyl derivative so obtained melts between 191 and 197.
C: Shake 0.1 g with 10 mL of water, and filter. To 5 mL of the filtrate add 1 drop of ferric chloride TS: a violet color is produced.
pH <791>: between 3.0 and 3.7, in a saturated solution.
Water, Method I <921>: not more than 0.5%.
Residue on ignition <281>: not more than 0.2%.
Chloride <221>- Dissolve 0.50 g in a mixture of 5 mL of nitric acid and 15 mL of water: the solution shows no more chloride than corresponds to 0.30 mL of 0.020 N hydrochloric acid (0.042%).
Heavy metals, Method II 231: 0.003%.
Limit of m-aminophenol-
Mobile phase- Prepare as directed in the Assay.
Internal standard solution- Prepare a solution of sulfanilamide in Mobile phase having a concentration of about 5 µg per mL.
Standard solution- Dissolve an accurately weighed quantity of USP m-Aminophenol RS in Mobile phase to obtain a solution having a known concentration of about 12 µg per mL. Transfer 10.0 mL of this solution and 10.0 mL of Internal standard solution to a 100-mL low-actinic volumetric flask, dilute with Mobile phase to volume, and mix.
Test solution- Transfer about 50 mg of Aminosalicylic Acid, accurately weighed, to a 100-mL low-actinic volumetric flask, add 50 mL of Mobile phase, and swirl to dissolve. Add 10.0 mL of Internal standard solution, dilute with Mobile phase to volume, and mix.
Chromatographic system (see Chromatography <621>)-The liquid chromatograph is equipped with a 280-nm detector and a 4.6-mm × 25-cm column that contains 10-µm packing L1. The flow rate is about 1.5 mL per minute. Chromatograph the Standard solution, and record the peak responses as directed for Procedure: the relative retention times are about 0.66 for sulfanilamide and 1.0 for m-aminophenol; the resolution, R, between m-aminophenol and sulfanilamide is not less than 2.5; and the relative standard deviation for replicate injections is not more than 7%.
Procedure- [note-After use, wash the column for 30 minutes with a filtered and degassed mixture of methanol, water, and phosphoric acid (77:23:0.6), and then wash for 30 minutes with a filtered and degassed mixture of methanol and water (50:50). ] Separately inject equal volumes (about 20 µL) of the Standard solution and the Test solution into the chromatograph, record the chromatograms, and measure the responses for the major peaks. Calculate the percentage of m-aminophenol, in relation to the quantity of aminosalicylic acid in the portion of Aminosalicylic Acid taken by the formula:
10(C / W)(RU / RS)
in which C is the concentration, in µg per mL, of USP m-Aminophenol RS in the Standard solution, W is the quantity of aminosalicylic acid, in mg, in the portion of Aminosalicylic Acid taken, as determined in the Assay; and RU and RS are the ratios of the response of the m-aminophenol peak to the response of the sulfanilamide peak obtained from the Test solution and the Standard solution; respectively: not more than 0.25% of m-aminophenol is found.
Hydrogen sulfide, sulfur dioxide, and amyl alcohol- Dissolve about 500 mg in 5 mL of 1 N sodium hydroxide, add 6 mL of 3 N hydrochloric acid, and stir vigorously: no odor of hydrogen sulfide or sulfur dioxide is perceptible, and not more than a faint odor of amyl alcohols is perceptible. A piece of moistened lead acetate test paper held over the mixture does not become discolored.
Assay-
Mobile phase- Prepare a mixture of 425 mL of 0.05 M dibasic sodium phosphate, 425 mL of 0.05 M monobasic sodium phosphate, and 150 mL of methanol containing 1.9 g of tetrabutylammonium hydroxide. Filter, and degas. Make adjustments if necessary (see System Suitability under Chromatography 621).
Internal standard solution- Prepare a solution of acetaminophen in Mobile phase having a concentration of about 5 mg per mL.
Standard preparation- Transfer about 12.5 mg of USP Aminosalicylic Acid RS, accurately weighed, to a 25-mL low-actinic volumetric flask, add 15 mL of Mobile phase, and swirl to dissolve. Add 2.5 mL of Internal standard solution, dilute with Mobile phase to volume, and mix.
Assay preparation- Prepare as directed for Standard preparation, except to use Aminosalicylic Acid instead of USP Aminosalicylic Acid RS.
Chromatographic system (see Chromatography <621>)-The chromatograph is equipped with a 254-nm detector and a 4.6-mm × 25-cm column that contains packing L1. The flow rate is about 1.5 mL per minute. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the relative retention times are about 0.83 for acetaminophen and 1.0 for aminosalicylic acid; the resolution, R, between aminosalicylic acid and acetaminophen is not less than 1.7; and the relative standard deviation of the ratios of the response of the aminosalicylic acid peak to the response of the acetaminophen peak is not more than 1.0%.
Procedure- [note-After use, wash the column for 30 minutes with a filtered and degassed mixture of methanol, water, and phosphoric acid (77:23:0.6), and then wash for 30 minutes with a filtered and degassed mixture of methanol and water (50:50).] Separately inject equal volumes (about 20 µL) of the Standard preparation and the Assay preparation into the chromatograph, record the chromatograms, and measure the responses for the major peaks. Calculate the quantity, in mg, of C7H7NO3 in the Aminosalicylic Acid taken by the formula:
25C(RU / RS)
in which C is the concentration, in mg per mL, of USP Aminosalicylic Acid RS in the Standard preparation; and RU and RS are the ratios of the response of the aminosalicylic acid peak to the response of the acetaminophen peak obtained from the Assay preparation and the Standard preparation, respectively.
Package: Bottle, Aluminum foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Keep the container tightly closed. Store in a cool, dry (2-8℃) and well-ventilated warehouse away from incompatible substances. Keep away from sunshine; avoid fire and heat sources; avoid moisture.
Shipping: Deliver to worldwide by air, by FedEx / DHL Express. Provide fast and reliable delivery.
How to Purchase? Please contact: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Russia, Korea, Japanese, Australia, etc.
Advantages? Superior quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
Risk Codes
R22 - Harmful if swallowed
R36 - Irritating to the eyes
R36/37/38 - Irritating to eyes, respiratory system and skin.
R45 - May cause cancer
R35 - Causes severe burns
R61 - May cause harm to the unborn child
Safety Description
S26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S37/39 - Wear suitable gloves and eye/face protection
S45 - In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
S53 - Avoid exposure - obtain special instructions before use.
S36 - Wear suitable protective clothing.
S36/37/39 - Wear suitable protective clothing, gloves and eye/face protection.
UN IDs UN 1789 8/PG 3
WGK Germany 2
RTECS VO1225000
TSCA Yes
HS Code 2918219000
4-Aminosalicylic Acid (4-ASA) (CAS: 65-49-6), an aminobenzoic acid that is salicylic acid substituted by an amino group at position 4, is one of the last remaining drugs available to treat extensively drug-resistant (XDR) tuberculosis. It is an antitubercular agent often administered in association with Isoniazid. 4-Aminosalicylic Acid exhibits anti-inflammatory, antioxidative, and antibacterial activities; it is clinically used to treat inflammatory bowel disease (IBD), Crohn’s disease, and colitis, as it acts primarily in the colon. It is typically taken by mouth. 4-Aminosalicylic Acid is used against Mycobacterium as a treatment for tuberculosis; it inhibits dihydrofolate reductase (DHFR) activity as an antimetabolite. 4-Aminosalicylic Acid, an antituberculosis drug, is a model active pharmaceutical ingredient to study salt and cocrystal formation in a multiple hydrogen-bonding functionality molecule with carboxylic acid, amine, and phenol groups. A antibacterial capable of inhibiting the growth of Mycobacterium tuberculosis.
-
4-Aminosalicylic Acid CAS 65-49-6 Assay 98.5%~1...
-
5-Aminosalicylic Acid CAS 89-57-6 (Mesalamine; ...
-
Salicylic Acid CAS 69-72-7 Purity >99.0% (HPLC)...
-
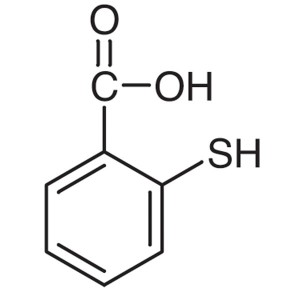
Thiosalicylic Acid CAS 147-93-3 (2-Mercaptobenz...
-
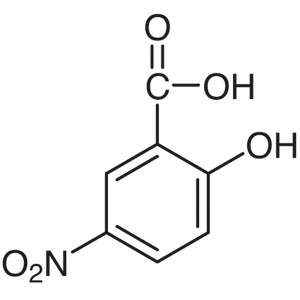
5-Nitrosalicylic Acid CAS 96-97-9 Purity >99.5%...
-
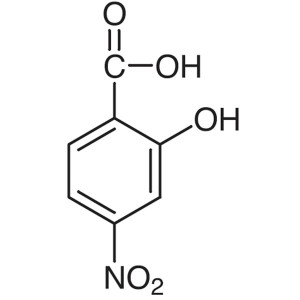
4-Nitrosalicylic Acid CAS 619-19-2 Purity >98.0...
-
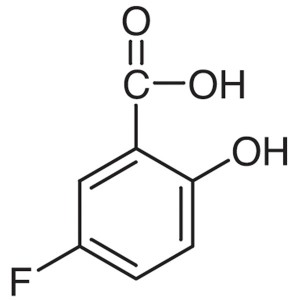
5-Fluorosalicylic Acid CAS 345-16-4 Purity >99....
-
4-Fluorosalicylic Acid CAS 345-29-9 Purity >99....
-
4-Bromosalicylic Acid CAS 1666-28-0 Purity >98....
-
3,5-Dinitrosalicylic Acid CAS 609-99-4 Purity >...
-
3-Methylsalicylic Acid CAS 83-40-9 Purity >98.0...
-
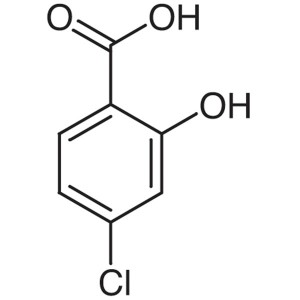
4-Chlorosalicylic Acid CAS 5106-98-9 Purity >98...
-
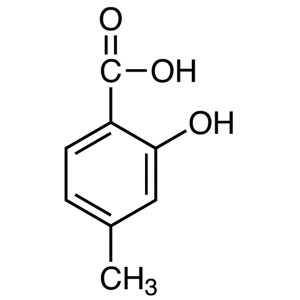
4-Methylsalicylic Acid CAS 50-85-1 Purity >99.0...
-
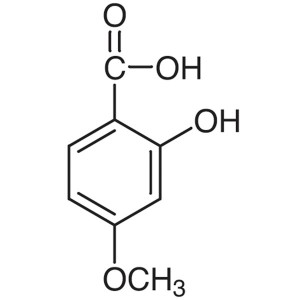
4-Methoxysalicylic Acid CAS 2237-36-7 Purity >9...
-
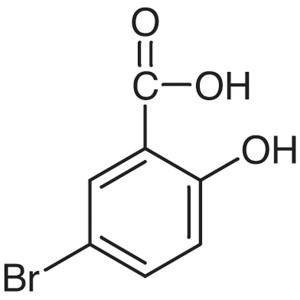
5-Bromosalicylic Acid CAS 89-55-4 Purity >98.0%...
-
5-Chlorosalicylic Acid CAS 321-14-2 Purity >99....