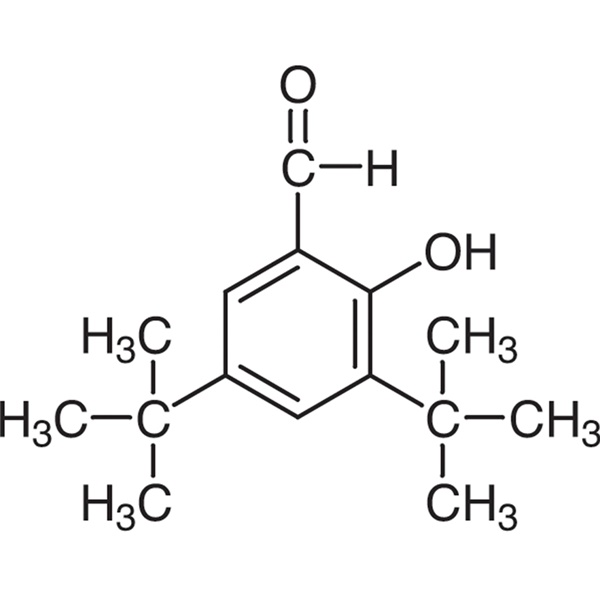
3,5-Di-tert-Butylsalicylaldehyde CAS 37942-07-7 Purity >99.0% (GC) Factory High Quality
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer and supplier of 3,5-Di-tert-Butylsalicylaldehyde (CAS: 37942-07-7) with high quality, commercial production. Welcomed to order.
| Chemical Name | 3,5-Di-tert-Butylsalicylaldehyde |
| Synonyms | 3,5-Di-tert-butyl-2-Hydroxybenzaldehyde |
| CAS Number | 37942-07-7 |
| CAT Number | RF-PI1615 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C15H22O2 |
| Molecular Weight | 234.34 |
| Density | 1.006±0.06 g/cm3 |
| Solubility | Soluble in Methanol |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | Light Yellow to Yellow Powder or Crystals |
| Purity / Analysis Method | >99.0% (GC) |
| Melting Point | 58.0~63.0℃ |
| Loss on Drying | <1.00% |
| Total Impurities | <1.00% |
| 1 H NMR Spectrum | Consistent With Structure |
| Test Standard | Enterprise Standard |
| Usage | Pharmaceutical Intermediates |
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture.


3,5-Di-tert-Butylsalicylaldehyde (CAS: 37942-07-7) can be used as an organic synthesis intermediate and pharmaceutical intermediate. 3,5-Di-tert-Butylsalicylaldehyde is used in the synthesis of Mn(III)-salen complex and its diamino precursor 5,6-diamino-5,6-dideoxy-1,2-O-isopropylidene-3-O-methyl-β-L-idofuranose, chiral Schiff base ligand for an enantioselective copper-catalyzed addition of phenyl acetylene to imines, chiral oxazolidine ligand for the enantioselective addition of diethyl zinc to aldehydes and tin Schiff base complexes with histidine analogues. It has antibacterial activity and is used in the preparation nickel complexes. It is structurally related to 3,5-di-t-Butylcatechol (DTCAT) but is not as potent an activator of rat skeletal muscle ryanodine receptor Ca2+ channel (RyRC).