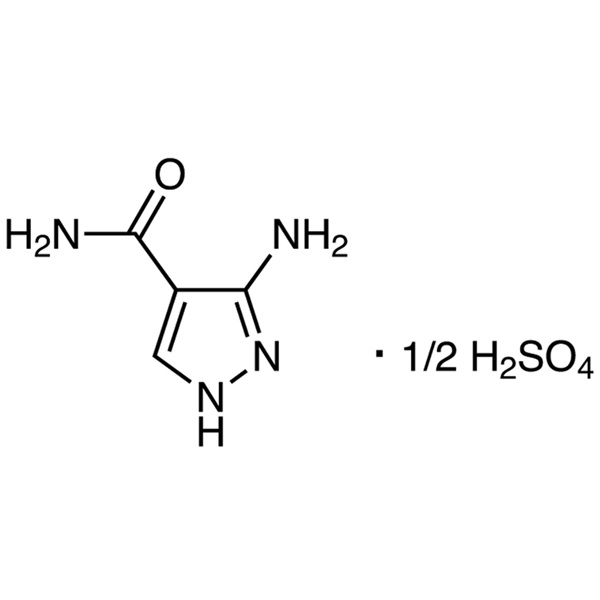
3-Amino-4-Pyrazolecarboxamide Hemisulfate CAS 27511-79-1 Purity >99.5% (HPLC) Factory
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer and supplier of 3-Amino-4-Pyrazolecarboxamide Hemisulfate (CAS: 27511-79-1) with high quality. We can provide COA, worldwide delivery, small and bulk quantities available. If you are interested in this product, please send detailed information includes CAS number, product name, quantity to us. Please contact: alvin@ruifuchem.com
| Chemical Name | 3-Amino-4-Pyrazolecarboxamide Hemisulfate |
| Synonyms | 3-Aminopyrazole-4-Carboxamide Hemisulfate; 3-Amino-4-Carbamoylpyrazole Hemisulfate; 3-Amino-4-Pyrazolecarboxamide Hemisulfate Salt; 3-Amino-1H-Pyrazole-4-Carboxamide Hemisulfate; 3-Amino-Pyrazole-4-Carboxamide Hemisulfate |
| Impurity | Allopurinol EP Impurity A; Allopurinol USP Related Compound A |
| CAS Number | 27511-79-1 |
| CAT Number | RF2578 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C4H6N4O·1/2H2SO4 |
| Molecular Weight | 175.16 |
| Melting Point | 224℃(dec.)(lit.) |
| Density | 0.84 |
| Sensitive | Hygroscopic |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | White to Off-White Powder |
| Purity / Analysis Method | >99.5% (HPLC) |
| Water (by Karl Fischer) | <1.00% |
| Residue on Ignition | <0.10% |
| Single Impurity | <0.50% |
| Total Impurities | <0.50% |
| Heavy Metals | <20ppm |
| Infrared Spectrum | Conforms to Structure |
| Proton NMR Spectrum | Conforms to Structure |
| Test Standard | Enterprise Standard |
| Usage | Impurity / Intermediate of Allopurinol (CAS: 315-30-0) |
Package: Bottle, Aluminum foil bag, 25kg/Cardboard Drum, or according to customer's requirement
Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture
How to Purchase? Please contact: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Russia, Korea, Japanese, Australia, etc.
Advantages? Excellent quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
3-Amino-4-Pyrazolecarboxamide Hemisulfate (CAS: 27511-79-1) is a pyrazole derivative, it's an Impurity / Intermediate of Allopurinol (CAS: 315-30-0). Allopurinol is the drug of choice in the treatment of chronic tophaceous gout and is especially useful in patients whose treatment is complicated by renal insufficiency. Allopurinol was synthesized in 1956 as part of a study of purine antagonists. It is well absorbed on oral administration, with peak plasma concentrations appearing within 1 hour. Decreases of uric acid can be observed within 24 to 48 hours. Excretion of allopurinol and its metabolite occurs primarily in the urine, with approximately 20% of a dose being excreted in the feces. Allopurinol does not reduce serum uric acid levels by increasing renal uric acid excretion; instead it lowers plasma urate levels by inhibiting the final steps in uric acid biosynthesis. This action is accomplished by inhibiting xanthine oxidase, the enzyme involved in the metabolism of hypoxanthine and xanthine to uric acid. After enzyme inhibition, the urinary and blood concentrations of uric acid are greatly reduced and there is a simultaneous increase in the excretion of the more soluble uric acid precursors, xanthine and hypoxanthine.
-
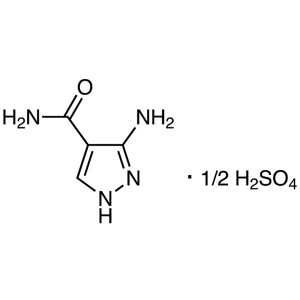
3-Amino-4-Pyrazolecarboxamide Hemisulfate CAS 2...
-
3-Amino-4-Pyrazolecarbonitrile CAS 16617-46-2 P...
-
Ethyl 3-Aminopyrazole-4-Carboxylate CAS 6994-25...
-
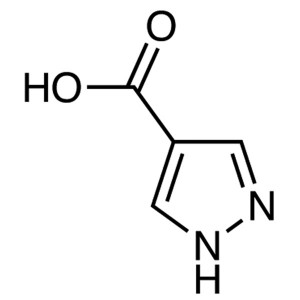
4-Pyrazolecarboxylic Acid CAS 37718-11-9 Purity...
-
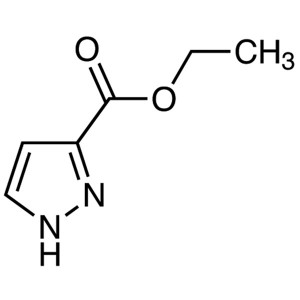
Ethyl 3-Pyrazolecarboxylate CAS 5932-27-4 Purit...
-
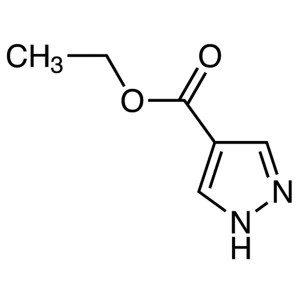
Ethyl 4-Pyrazolecarboxylate CAS 37622-90-5 Puri...
-
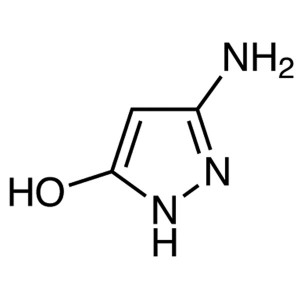
3-Amino-5-Hydroxypyrazole CAS 6126-22-3 Purity ...
-
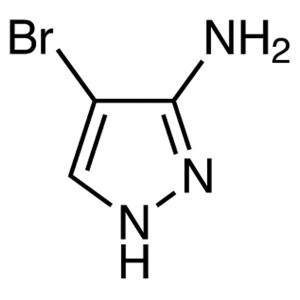
3-Amino-4-Bromopyrazole CAS 16461-94-2 Purity >...
-
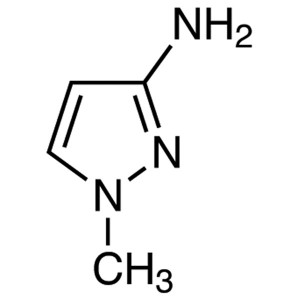
3-Amino-1-Methylpyrazole CAS 1904-31-0 Purity >...
-
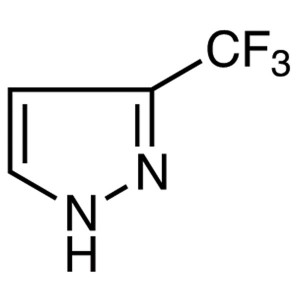
3-(Trifluoromethyl)pyrazole CAS 20154-03-4 Puri...
-
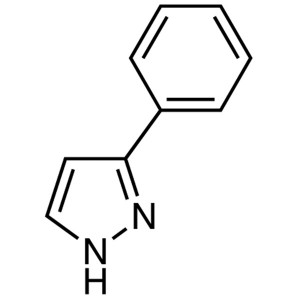
3-Phenylpyrazole CAS 2458-26-6 Purity >98.0% (H...
-
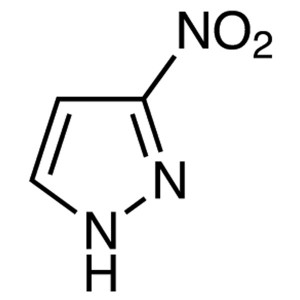
3-Nitropyrazole CAS 26621-44-3 Purity >99.0% (H...
-
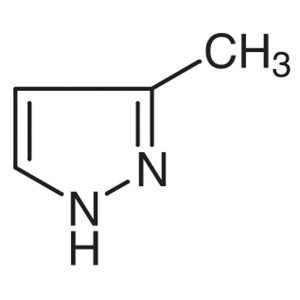
3-Methylpyrazole CAS 1453-58-3 Purity >99.0% (G...
-
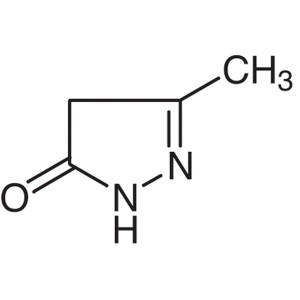
3-Methyl-5-Pyrazolone CAS 108-26-9 Purity >98.0...
-
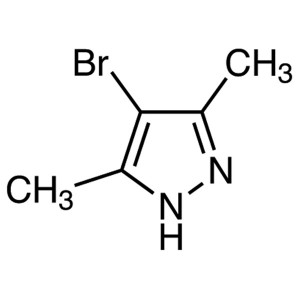
4-Bromo-3,5-Dimethylpyrazole CAS 3398-16-1 Puri...
-
4-Bromo-1-Methylpyrazole CAS 15803-02-8 Purity ...