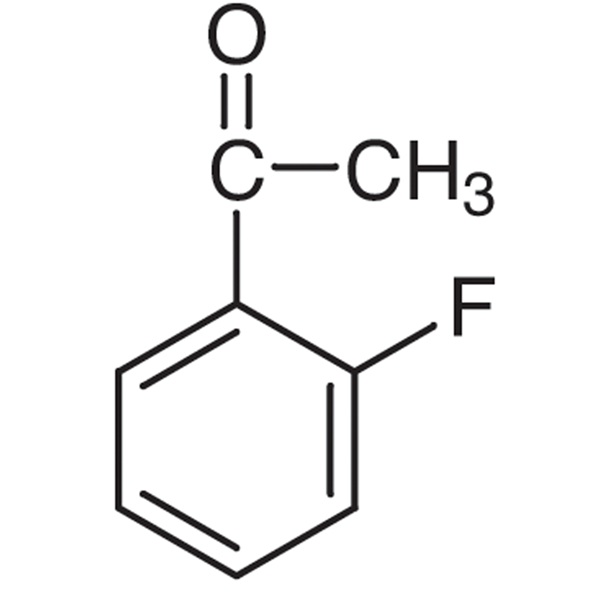
2′-Fluoroacetophenone CAS 445-27-2 Purity >98.0% (GC)
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer and supplier of 2'-Fluoroacetophenone (CAS: 445-27-2) with high quality. We can provide COA, worldwide delivery, small and bulk quantities available. If you are interested in this product, please send detailed information includes CAS number, product name, quantity to us. Please contact: alvin@ruifuchem.com
| Chemical Name | 2'-Fluoroacetophenone |
| Synonyms | o-Fluoroacetophenone; 2-Fluoroacetophenone; 1-(2-Fluorophenyl)ethanone; 1-(2-Fluorophenyl)ethan-1-one |
| CAS Number | 445-27-2 |
| CAT Number | RF2963 |
| Stock Status | In Stock, Production Capacity 20 Tons per Month |
| Molecular Formula | C8H7FO |
| Molecular Weight | 138.14 |
| Boiling Point | 82.0~83.0℃/10 mmHg |
| Flash Point | 61℃(141°F) |
| Solubility | Soluble in Acetone, Chloroform, Dichloromethane, Ethanol, Ethyl Acetate and Methanol |
| Hazard Class | Irritant |
| Packing Group | III |
| HS Code | 29147090 |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | Colorless to Light Yellow Liquid |
| Purity / Analysis Method | >98.0% (GC) |
| Density (20℃) | 1.139~1.142 |
| Refractive Index n20/D | 1.507~1.509 |
| 1 H NMR Spectrum | Consistent With Structure |
| LCMS | Consistent With Structure |
| Water (by Karl Fischer) | <0.50% |
| Total Impurities | <2.00% |
| Test Standard | Enterprise Standard |
| Usage | Pharmaceutical Intermediate; Vonoprazan Fumarate Intermediate / Impurity |
Package: Fluorinated Bottle, 25kg/Drum, 200kg/Drum, or according to customer's requirement
Storage Condition: Store in sealed containers at cool and dry place. Protect from light and moisture. Store away from strong oxidizing agents.
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc.
Advantages? Superior quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
2'-Fluoroacetophenone (CAS: 445-27-2) is a pharmaceutical intermediate, which can be used as a starting material to synthesize Vonoprazan Fumarate (CAS: 1260141-27-2). Vonoprazan Fumarate (Takecab®), discovered and developed by Takeda and Otsuka, was approved by the PMDA of Japan in December 2014, and is indicated for the treatment of gastric ulcer, duodenal ulcer and reflux esophagitis. Vonoprazan Fumarate has a novel mechanism of action called potassium-competitive acid blockers, which competitively inhibit the binding of potassium ions to H+, K+-ATPase (also known as the proton pump) in the final step of gastric acid secretion in gastric parietal cells. Vonoprazan does not inhibit Na+, K+-ATPase activity even at concentrations 500 times higher than that of their IC50 values against gastric H+, K+-ATPase activity. Furthermore, the drug is unaffected by the gastric secretory state, unlike PPIs.
-
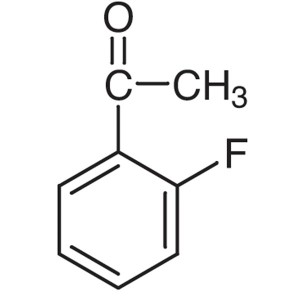
2′-Fluoroacetophenone CAS 445-27-2 Purity...
-
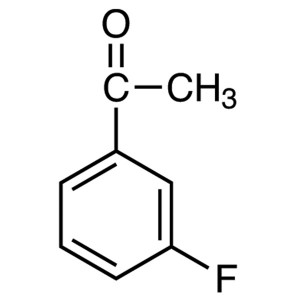
3′-Fluoroacetophenone CAS 455-36-7 Purity...
-
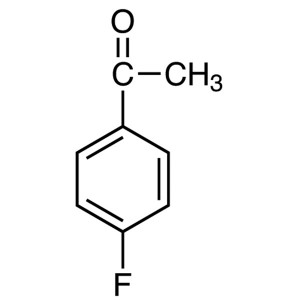
4′-Fluoroacetophenone CAS 403-42-9 Purity...
-
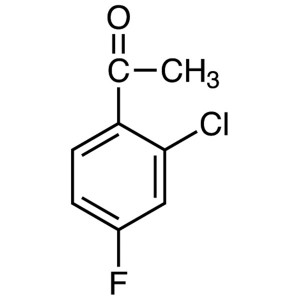
2′-Chloro-4′-Fluoroacetophenone CAS...
-
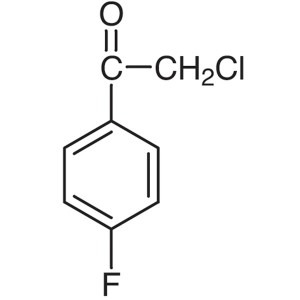
2-Chloro-4′-Fluoroacetophenone CAS 456-04...
-
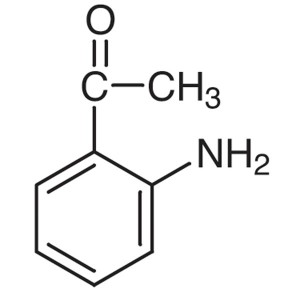
2′-Aminoacetophenone CAS 551-93-9 Purity ...
-
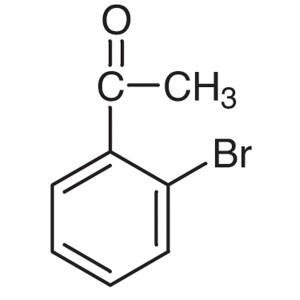
2′-Bromoacetophenone CAS 2142-69-0 Purity...
-
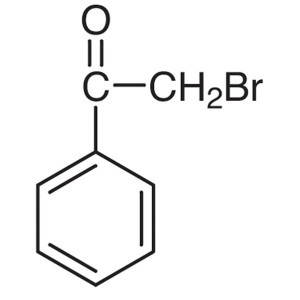
2-Bromoacetophenone CAS 70-11-1 (Phenacyl Bromi...
-
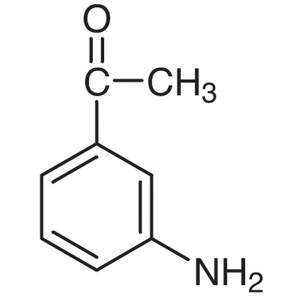
3′-Aminoacetophenone CAS 99-03-6 Purity >...
-
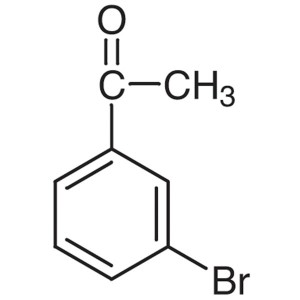
3′-Bromoacetophenone CAS 2142-63-4 Purity...
-
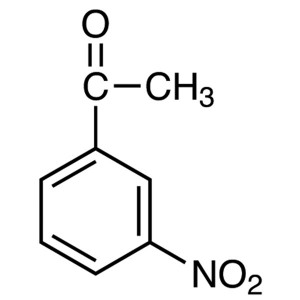
3′-Nitroacetophenone CAS 121-89-1 Purity ...
-
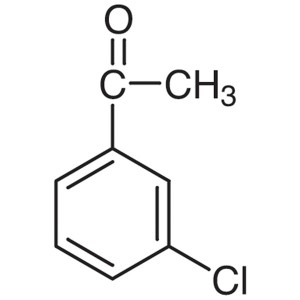
3′-Chloroacetophenone CAS 99-02-5 Purity ...
-
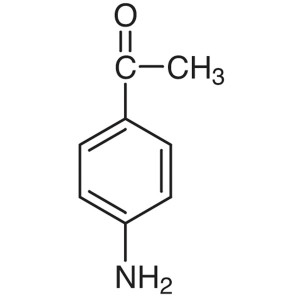
4′-Aminoacetophenone CAS 99-92-3 Purity >...
-
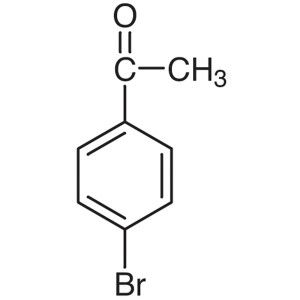
4′-Bromoacetophenone CAS 99-90-1 Purity >...
-
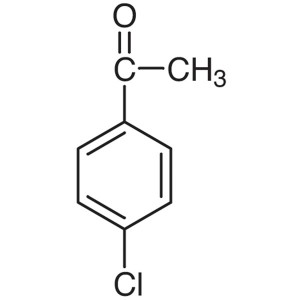
4′-Chloroacetophenone CAS 99-91-2 Purity ...