2-Fluoro-5-Formylbenzonitrile CAS 218301-22-5 Purity ≥98.0% Olaparib Intermediate Factory
High Purity, Commercial Production
Olaparib and Related Intermediates:
Olaparib CAS 763113-22-0
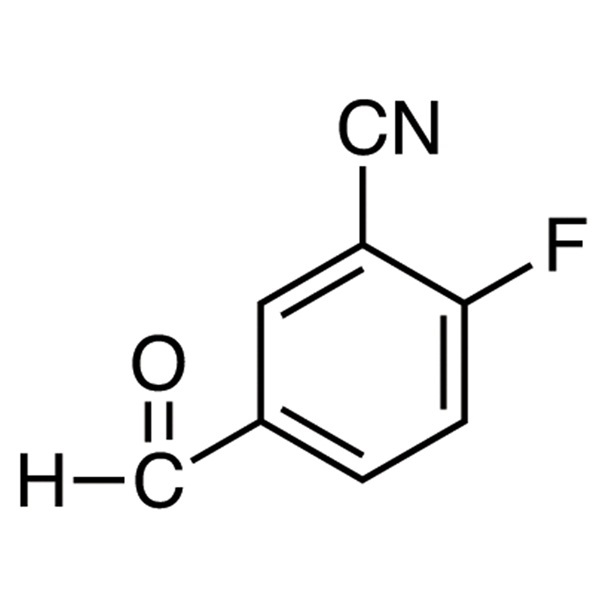
2-Fluoro-5-Formylbenzonitrile CAS 218301-22-5
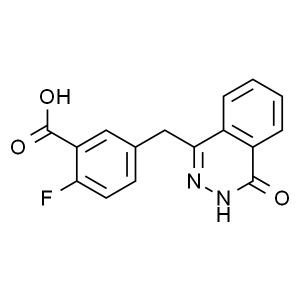
2-Fluoro-5-((4-oxo-3,4-dihydrophthalazin-1-yl)methyl)benzoic acid CAS 763114-26-7
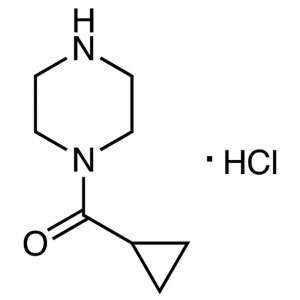
1-(Cyclopropylcarbonyl)piperazine Hydrochloride CAS 1021298-67-8
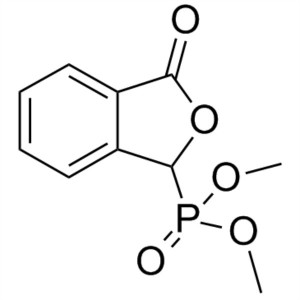
3-Oxo-1,3-Dihydroisobenzofuran-1-Ylphosphonic Acid CAS 61260-15-9
| Chemical Name | 2-Fluoro-5-Formylbenzonitrile |
| Synonyms | 3-Cyano-4-Fluorobenzaldehyde |
| CAS Number | 218301-22-5 |
| CAT Number | RF-PI451 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C8H4FNO |
| Molecular Weight | 149.12 |
| Melting Point | 80.0 to 84.0℃ (lit.) |
| Solubility | Soluble in Methanol |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | Yellow to Off-white Powder |
| Purity | ≥98.0% |
| Moisture (K.F) | ≤0.50% |
| Total Impurities | ≤2.0% |
| Test Standard | Enterprise Standard |
| Usage | Intermediate of Olaparib (CAS: 763113-22-0) PARP-Inhibitor |
Package: Bottle, Aluminium foil bag, Cardboard Drum, 25kg/Drum, or according to customer's requirement.
Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture.


2-Fluoro-5-Formylbenzonitrile (CAS: 218301-22-5) is used in the preparation of heterocyclic compounds as PARP inhibitors for medical use. 2-Fluoro-5-Formylbenzonitrile is used as an intermediate of Olaparib (CAS: 763113-22-0). Olaparib is a small molecule inhibitor of PARP1/PARP2 (IC50: 5/1 nM) but is less effective against the PARP tankyrase-1 (IC50: 1.5 µM). Olaparib (AZD-2281, trade name Lynparza) is an FDA-approved targeted therapy for cancer, developed by KuDOS Pharmaceuticals and later by AstraZeneca. It is a PARP inhibitor, inhibiting poly ADP ribose polymerase (PARP), an enzyme involved in DNA repair.It acts against cancers in people with hereditary BRCA1 or BRCA2 mutations, which include some ovarian, breast, and prostate cancers. In December 2014, olaparib was approved for use as a single agent by the EMA and the FDA. The FDA approval is in for germline BRCA mutated (gBRCAm) advanced ovarian cancer that has received three or more prior lines of chemotherapy.