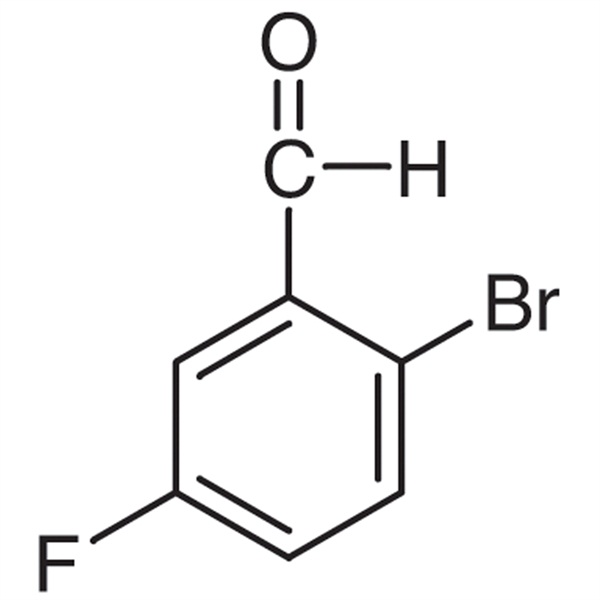
2-Bromo-5-Fluorobenzaldehyde CAS 94569-84-3 Assay ≥98.0% Factory High Quality
Manufacturer Supply with High Purity and Stable Quality
Chemical Name: 2-Bromo-5-Fluorobenzaldehyde
CAS: 94569-84-3
High Quality, Commercialized Production
| Chemical Name | 2-Bromo-5-Fluorobenzaldehyde |
| CAS Number | 94569-84-3 |
| CAT Number | RF-PI325 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C7H4BrFO |
| Molecular Weight | 203.01 |
| Density | 1.7±0.1 g/cm3 |
| Boiling Point | 225.8±20.0℃ at 760 mmHg |
| Solubility | Soluble in Methanol |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | Light Yellow Crystal |
| Water | <0.50% |
| Melting Point | 51.0~56.0℃ |
| Assay | ≥98.0% |
| Test Standard | Enterprise Standard |
| Usage | Pharmaceutical Intermediates |
Package: Bottle, Aluminum foil bag, Cardboard drum, 25kg/Drum, or according to customer's requirement.
Storage Condition: Store in sealed containers at cool and dry place; Protect from light, moisture and pest infestation.


Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer and supplier of 2-Bromo-5-Fluorobenzaldehyde (CAS: 94569-84-3) with high quality, widely used in organic synthesis, synthesis of pharmaceutical intermediates and Active Pharmaceutical Ingredient (API) synthesis. 2-Bromo-5-Fluorobenzaldehyde is used as Tavaborole intermediate and has been used as a reactant for the preparation of pyridopyrimidinediones. 2-Bromo-5-fluorobenzaldehyde is used as a precursor for the synthesis of 5-fluoro-3-substituted benzoxaboroles, which is used in material science as molecular receptors, building block in crystal engineering, as steroid conjugates for molecular imprinting, dyes and biosensors of alpha hydroxyl carboxylic acids. It is also used in the synthesis of 5-arylindazolo[3,2-b]quinazolin-7(5H)-one by reacting with 2-amino-N′-arylbenzohydrazide in the presence of Copper(I) bromide by the Ullmann-type reaction. 2-Bromo-5-fluorobenzaldehyde can be prepared by reacting 2-bromo-5-fluorotoluene with N-bromosuccinimide. Its crystals exhibit monoclinic crystal system and space group P21/c.
-
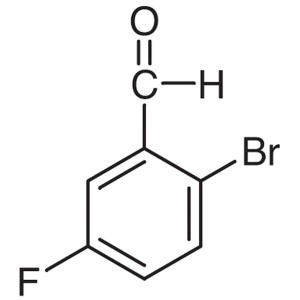
2-Bromo-5-Fluorobenzaldehyde CAS 94569-84-3 Ass...
-
5-Bromo-2-Fluorobenzaldehyde CAS 93777-26-5 Hig...
-
2-Bromo-6-Fluorobenzaldehyde CAS 360575-28-6 Hi...
-
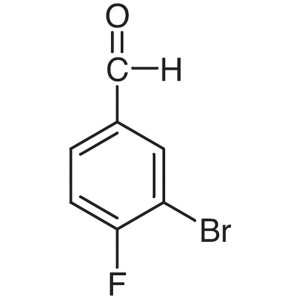
3-Bromo-4-Fluorobenzaldehyde CAS 77771-02-9 Ass...
-
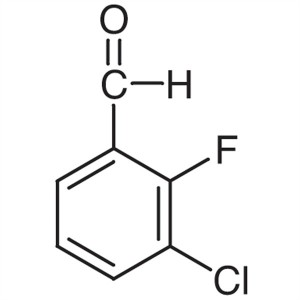
3-Chloro-2-Fluorobenzaldehyde CAS 85070-48-0 As...
-
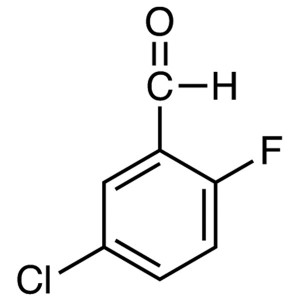
5-Chloro-2-Fluorobenzaldehyde CAS 96515-79-6 Hi...