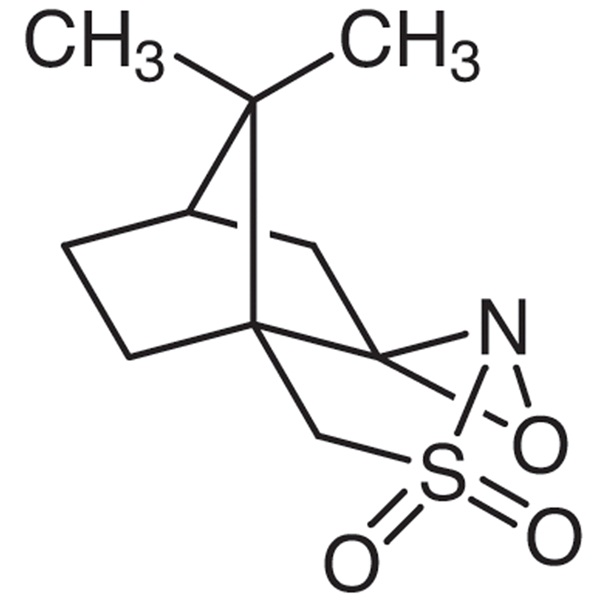
(1S)-(+)-(10-Camphorsulfonyl)oxaziridine CAS 104322-63-6 Purity ≥98.5% (HPLC) High Purity
Manufacturer Supply with High Purity and Stable Quality
(1S)-(+)-(10-Camphorsulfonyl)oxaziridine CAS 104322-63-6
(1R)-(-)-(10-Camphorsulfonyl)oxaziridine CAS 104372-31-8
Chiral Compounds, High Quality, Commercial Production
| Chemical Name | (1S)-(+)-(10-Camphorsulfonyl)oxaziridine |
| Synonyms | (1S)-(+)-(Camphorylsulfonyl)oxaziridine; (2R,8aS)-(+)-(Camphorylsulfonyl)oxaziridine |
| CAS Number | 104322-63-6 |
| CAT Number | RF-CC267 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C10H15NO3S |
| Molecular Weight | 229.3 |
| Shipping Condition | Shipped Under Ambient Temperature |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | Off-White or Pale Yellow Crystalline Powder |
| Purity | ≥98.5% (HPLC) |
| Melting Point | 168.0~172.0℃ |
| Specific Rotation [a]D20 | +43.0° ~ +47.0° (C=2.3, In CHCl3) |
| Loss on Drying | ≤1.0% |
| Residue on Ignition | ≤0.30% |
| Heavy Metals (Pb) | ≤20ppm |
| Test Standard | Enterprise Standard |
| Usage | Chiral Compounds; Pharmaceutical Intermediates |
Package: Bottle, Aluminium foil bag, Cardboard Drum, 25kg/Drum, or according to customer's requirement.
Storage Condition: Store in sealed containers at cool and dry place; Protect from light, moisture and pest infestation.


Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer and supplier of (1S)-(+)-(10-Camphorsulfonyl)oxaziridine (CAS: 104322-63-6) with high quality.
(1S)-(+)-(10-Camphorsulfonyl)oxaziridine (CAS: 104322-63-6) is a useful Camphor derivative, a useful synthetic intermediate. Used for asymmetric hydroxylation.
(1S)-(+)-(10-Camphorsulfonyl)oxaziridine (CAS: 104322-63-6) can be used:
To convert prochiral ketone enolates into optically active α-hydroxy ketones via enantioselective asymmetric oxidation.
In the synthesis of thymidine oligonucleotides connected through pyrophosphates.
In the asymmetric synthesis of proton pump inhibitors like (R)-Rabeprazole sodium and (R)-Lansoprazole sodium from the corresponding DBU salt of prochiral sulfide.
In the preparation of phosphonoacetate and thiophosphonoacetate oligodeoxynucleotides by oxidizing the corresponding phosphinoacetate.
-
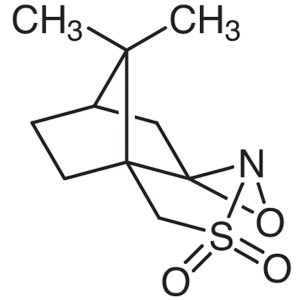
(1S)-(+)-(10-Camphorsulfonyl)oxaziridine CAS 10...
-
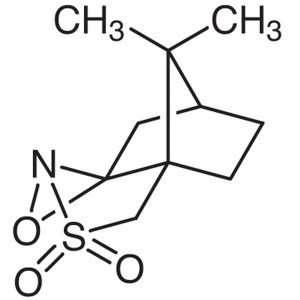
(1R)-(-)-(10-Camphorsulfonyl)oxaziridine CAS 10...
-
(±)-Camphor (Synthetic) CAS 76-22-2 Assay ≥99.0...
-
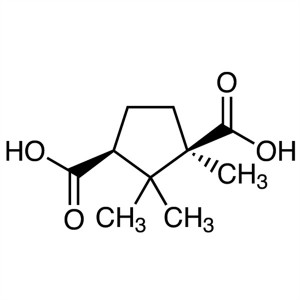
D-(+)-Camphoric Acid CAS 124-83-4 Purity 99.0%~...
-
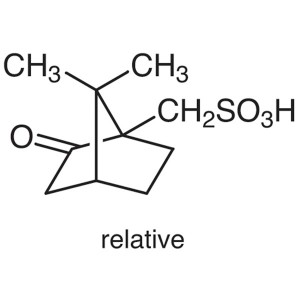
DL-10-Camphorsulfonic Acid CAS 5872-08-2 Assay ...
-
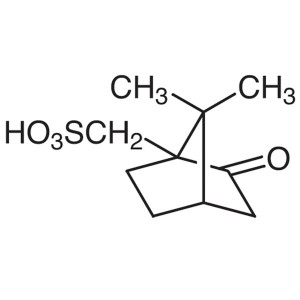
(1R)-(-)-10-Camphorsulfonic Acid CAS 35963-20-3...
-
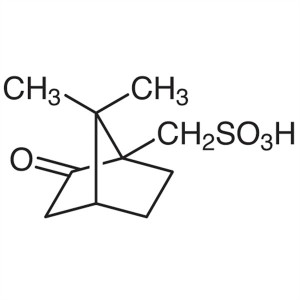
(1S)-(+)-10-Camphorsulfonic Acid CAS 3144-16-9 ...
-
(1S)-(-)-Camphanic Acid CAS 13429-83-9 Purity ≥...
-
(1R)-(+)-Camphanic Acid CAS 67111-66-4 Purity ≥...
-
(-)-10,2-Camphorsultam CAS 94594-90-8 Assay ≥98...
-
(+)-10,2-Camphorsultam CAS 108448-77-7 Assay ≥9...
-
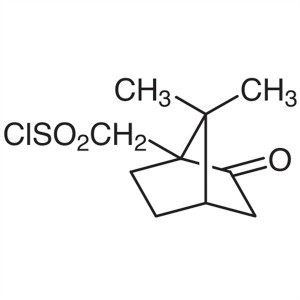
(1R)-(-)-10-Camphorsulfonyl Chloride CAS 39262-...
-
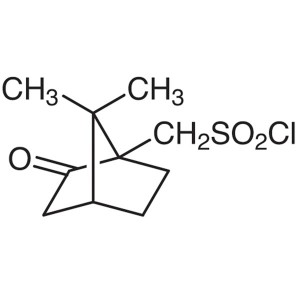
(1S)-(+)-10-Camphorsulfonyl Chloride CAS 21286-...
-
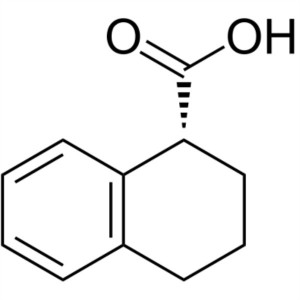
(R)-1,2,3,4-Tetrahedro-1-Naphthoic Acid CAS 233...
-
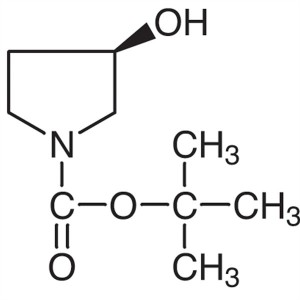
(R)-1-Boc-3-Hydroxypyrrolidine CAS 109431-87-0 ...
-
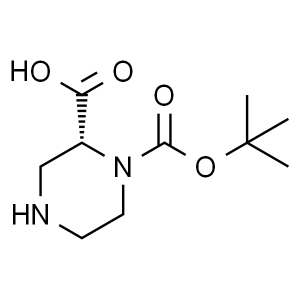
(R)-1-Boc-Piperazine-2-Carboxylic Acid CAS 2787...