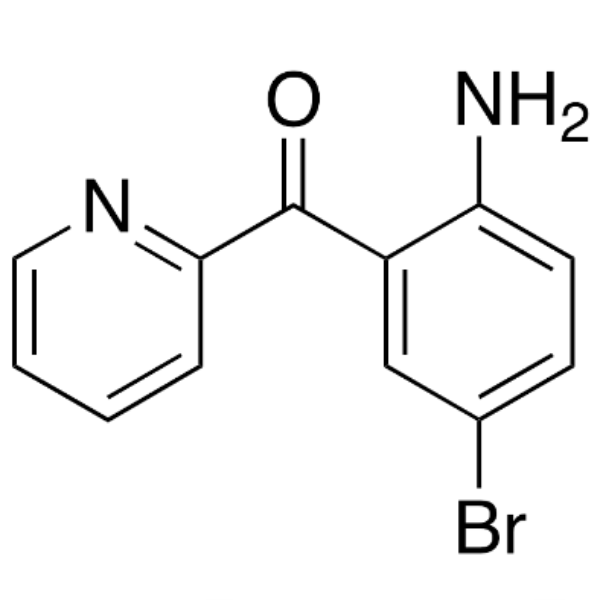
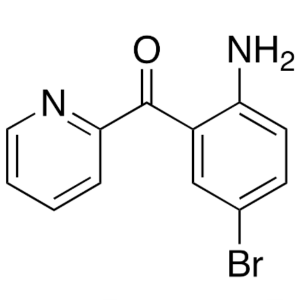
2-(2-Amino-5-Bromobenzoyl)pyridine CAS 1563-56-0 Purity >99.0% (HPLC) Factory
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer of 2-(2-Amino-5-Bromobenzoyl)pyridine (CAS: 1563-56-0) with high quality, intermediate of Remimazolam & Bromazepam. Ruifu Chemical can provide worldwide delivery, competitive price, excellent service, small and bulk quantities available. Purchase Remimazolam Intermediates, Please contact: alvin@ruifuchem.com
| Chemical Name | 2-(2-Amino-5-Bromobenzoyl)pyridine |
| Synonyms | 2-Amino-5-Bromobenzoylpyridine; 4-Bromo-2-[(Pyridin-2-yl)carbonyl]aniline; (2-Amino-5-Bromophenyl)(Pyridin-2-yl)methanone; 2-Amino-5-Bromophenyl 2-Pyridyl Ketone; 2-Amino-5-Bromophenyl-2-Pyridylmethanone |
| Impurity | Bromazepam EP impurity A |
| Stock Status | In Stock, Commercial Production |
| CAS Number | 1563-56-0 |
| Molecular Formula | C12H9BrN2O |
| Molecular Weight | 277.12 g/mol |
| Melting Point | 97.0~101.0℃ |
| Boiling Point | 451℃ |
| Flash Point | 227℃ |
| Density | 1.546 |
| Refractive Index n20/D | 1.658 |
| COA & MSDS | Available |
| Origin of Product | Shanghai, China |
| Product Categories |
Pharmaceutical Intermediates |
| Brand | Ruifu Chemical |
| Items | Specifications | Results |
| Appearance | Yellow Crystalline Powder | Yellow Crystalline Powder |
| Melting Point | 97.0~101.0℃ | 97.3~98.3℃ |
| Loss on Drying | <0.50% | 0.17% |
| Purity / Analysis Method | >99.0% (HPLC Area) | 99.7% |
| 1H NMR Spectrum | Conforms to Structure | Conforms |
| Conclusion | The product has been tested and complies with the given specifications | |
| Application | Intermediate of Remimazolam & Bromazepam | |
(1), Product Specifications
Properties: This product is yellow crystalline powder
Melting Point: 97.0~101.0℃
Loss on Drying: <0.50%
Purity Determination: This product is calculated on the dry basis, the purity shall not be less than 99.0%
(2), Method of Analysis
1. Properties: This product is yellow crystalline powder (visual)
2. Melting point: The melting point of this product (Chinese Pharmacopoeia 2005 Appendix VI C) is 97.0~101.0℃; The heating rate is 1.5℃/ min
3. Loss on Drying: Take 1g of this product, dry it at 80℃ for 4 hours, and the weight loss shall not exceed 0.50% (Chinese Pharmacopoeia 2005 Appendix VIII L)
4. Take the appropriate amount of this product, prepare 0.5mg/ml solution with flow matching, the sample size is 5μL, determine according to high performance liquid chromatography (Chinese Pharmacopoeia 2005 Appendix V D), calculate by area normalization method, and obtain.
5. Chromatographic Conditions (HPLC)
Instrument Model: Shimadzu
Column Type: C18 250*4.6mm
Mobile Phase: Methanol: 0.1% phosphoric acid aqueous solution (triethylamine adjusted pH value=3.4)=7:3
Sensitivity: 0.02 AUFA
Wavelength: 240nm
Flow Rate: 1.0ml/min
Column Temperature: 40℃
Retention Time: 30min
Package: Fluorinated Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Keep the container tightly closed and store in a cool, dry, well-ventilated warehouse away from incompatible substances. Keep away from sunshine; avoid fire and heat sources; avoid moisture.
Shipping: Deliver to worldwide by air, by FedEx / DHL Express. Provide fast and reliable delivery.
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc.
Advantages? Superior quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
2-(2-Amino-5-Bromobenzoyl)pyridine (CAS: 1563-56-0) is an intermediate of Remimazolam and Bromazepam.
Remimazolam, also known as CNS-7056, is a benzodiazepine derivative drug, developed by PAION, in collaboration with Japanese licensee Ono Pharmaceutical as an alternative to the short-acting imidazobenzodiazepine midazolam, for use in induction of anaesthesia and conscious sedation for minor invasive procedures. Remimazolam was found to be both faster acting and shorter lasting than midazolam, and human clinical trials showed a faster recovery time and predictable, consistent pharmacokinetics, suggesting some advantages over existing drugs for these applications.