Factory wholesale Cefotiam HCL - Palonosetron Hydrochloride CAS 135729-62-3 Purity ≥99.0% API USP EP High Quality – Ruifu
Factory wholesale Cefotiam HCL - Palonosetron Hydrochloride CAS 135729-62-3 Purity ≥99.0% API USP EP High Quality – Ruifu Detail:
Manufacturer with High Quality and Competitive Price
Commercial Supply Palonosetron Hydrochloride (CAS: 135729-62-3) Related Intermediates:
1,2,3,4-tetrahydro-naphthoic acid CAS: 1914-65-4
(S)-1,2,3,4-tetrahydro-naphthoic acid CAS: 85977-52-2
(R)-1,2,3,4-Tetrahedro-naphthoic acid CAS: 23357-47-3
(S)-3-Aminoquinuclidine dihydrochloride CAS: 119904-90-4
3-Quinuclidinone Hydrochloride CAS: 1193-65-3
1-Naphthoic acid CAS: 86-55-5
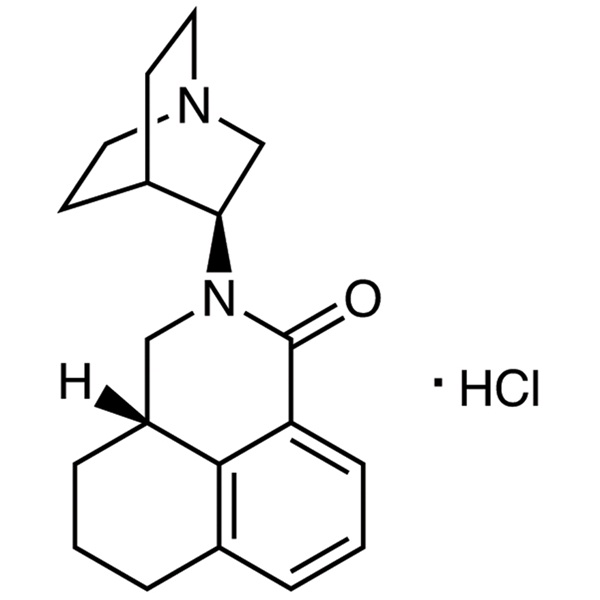
Palonosetron Hydrochloride CAS: 135729-62-3 API
| Chemical Name | Palonosetron Hydrochloride |
| Synonyms | Palonosetron HCl |
| CAS Number | 135729-62-3 |
| CAT Number | RF-API03 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C19H24N2O·HCl |
| Molecular Weight | 332.87 |
| Condition to Avoid | Heat Sensitive |
| Melting Point | 307 °C(dec.) |
| Solubility in Water | Completely Soluble |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | White or Off-White Crystalline Powder |
| Purity | ≥99.0% |
| Specific Rotation | -98°~-104° |
| Identification | Add BiI7K4,Generate Orange Yellow Precipitate |
| Identification | TLC Consistent with the Reference Substance |
| Identification | IR Consistent with Standard IR |
| Identification | Identification as Chloride |
| pH Vaule | 4.5~6.5 |
| Diastereoisomer (R,S)&(S,R) | ≤0.30% |
| Enantiomer (R,R) | ≤0.50% |
| Total Isomer | ≤0.50% |
| Tetrahydrofuran | ≤5000ppm |
| DMF | ≤880ppm |
| Normal Hexane | ≤290ppm |
| Methyl Alcohol | ≤3000ppm |
| Isopropanol | ≤5000ppm |
| Methylbenzene | ≤890ppm |
| Loss on Drying | ≤1.0% |
| Residue On Ignition | ≤0.10% |
| Heavy Metals | ≤10ppm |
| Test Standard | Enterprise Standard; United States Pharmacopoeia (USP);European Pharmacopoeia (EP) |
| Usage | Palonosetron Hydrochloride (CAS: 135729-62-3) API |
Package: Bottle, Cardboard Drum, 25kg/Drum, or according to customer’s requirement.
Storage Condition: Store in sealed containers at cool and dry place; Protect from light, moisture and pest infestation.


Manufacturer with High Quality and Competitive Price
Commercial Supply Palonosetron Hydrochloride (CAS: 135729-62-3) Related Intermediates:
1,2,3,4-tetrahydro-naphthoic acid CAS: 1914-65-4
(S)-1,2,3,4-tetrahydro-naphthoic acid CAS: 85977-52-2
(R)-1,2,3,4-Tetrahedro-naphthoic acid CAS: 23357-47-3
(S)-3-Aminoquinuclidine dihydrochloride CAS: 119904-90-4
3-Quinuclidinone Hydrochloride CAS: 1193-65-3
1-Naphthoic acid CAS: 86-55-5
Palonosetron Hydrochloride CAS: 135729-62-3 API
Palonosetron Hydrochloride CAS: 135729-62-3 is a drug to inhibit nausea and vomiting, which is a new type highly selective, high-affinity 5-HT3 receptor antagonist that can block the vomiting reflex center peripheral neurons presynaptic 5-HT. Receptor excitement directly affects the central nervous system in 5-HT. Impulse receptor excitement generated by the role of vagal afferent nerve after area, blocking the vagus nerve endings in the gut, preventing signals to 5-HT. Receptor trigger zone can reduce the incidence of nausea and vomiting. Central clinically for the treatment of acute severe emetogenic chemotherapy induced delayed nausea and vomiting. Because of its high curative effect, little side effect, long half-life (about 40 h), small dosage, and so on, it has attracted much attention.
With 1,2,3,4-tetrahydro-1-naphthoic acid as the starting material, the split, amination, reduction, cyclization, salt of palonosetron hydrochloride granisetron. Clinical studies showed that palonosetron can be used safely with corticosteroids, analgesics, antiemetics, antispasmodics and anticholinergic drugsas the starting material, the split, amination, reduction, cyclization, salt of palonosetron hydrochloride granisetron.
The most common adverse effects are headache, which occurs in 4–11% of patients, and constipation in up to 6% of patients. In less than 1% of patients, other gastrointestinal disorders occur, as well as sleeplessness, first-and second-degree atrioventricular block, muscle pain and shortness of breath. Palonosetron is similarly well tolerated as other setrons, and slightly less than placebo.
Product detail pictures:
Related Product Guide:
The incredibly rich projects administration experiences and a person to 1 service model make the substantial importance of organization communication and our easy understanding of your expectations for Factory wholesale Cefotiam HCL - Palonosetron Hydrochloride CAS 135729-62-3 Purity ≥99.0% API USP EP High Quality – Ruifu , The product will supply to all over the world, such as: Southampton, Kyrgyzstan, Monaco, With its rich manufacturing experience, high-quality products, and perfect after-sale service, the company has gained good reputation and has become one of the famous enterprise specialized in manufacturing series.We sincerely hope to establish business relation with you and pursue mutual benefit.
-
Low price for 5-FU - 3-Nitrobenzaldehyde CAS 9...
-
Excellent quality (S)-(+)-Benzyl Glycidyl Ether...
-
Chinese Professional D-(-)-Tartaric Acid Diethy...
-
Hot-selling Uracil - 2-(Bromomethyl)pyridine H...
-
OEM/ODM Manufacturer Dacarbazine Intermediate -...
-
Good quality D-2-Abu-NH2·HCl - (1R)-(+)-Campha...
Goods just received, we are very satisfied, a very good supplier, hope to make persistent efforts to do better.
