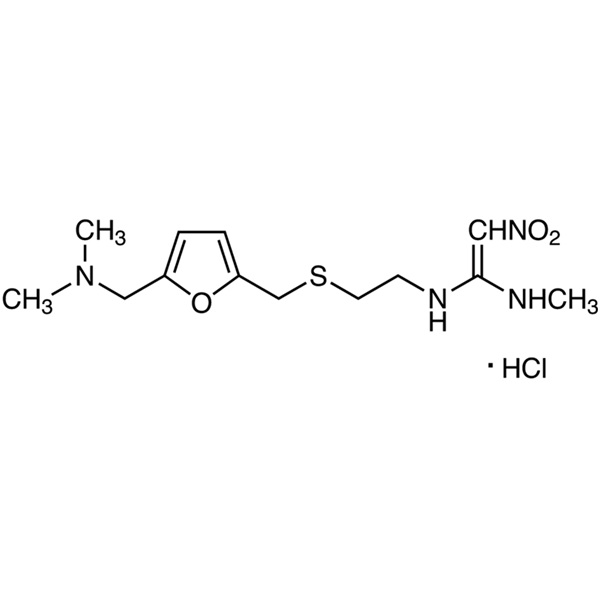
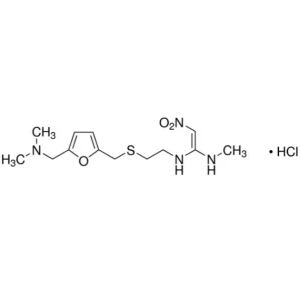
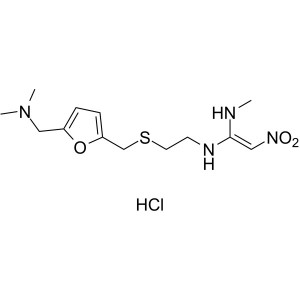
Ranitidine Hydrochloride CAS 66357-59-3 Assay 97.5~102.0%
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer of Ranitidine Hydrochloride (CAS: 66357-59-3) with high quality. Ruifu Chemical can provide worldwide delivery, competitive price, excellent service, small and bulk quantities available. Purchase Ranitidine Hydrochloride, Please contact: alvin@ruifuchem.com
| Chemical Name | Ranitidine Hydrochloride |
| Synonyms | Ranitidine HCl; Zantac; Zantadin; Zintac; Noctone; N-[2-[5-[(Dimethylamino)methyl]furfurylthio]ethyl]-N'-Methyl-2-Nitro-1,1-Ethenediamine Hydrochloride; N,N Dimethyl-5-[2-(1-Methylamine-2-Nitrovinyl)-Ethylthiomethyl]furfurylamine Hydrochloride |
| Stock Status | In Stock, Commercial Production |
| CAS Number | 66357-59-3 |
| Related CAS | 71130-06-8 |
| Molecular Formula | C13H22N4O3S·HCl |
| Molecular Weight | 350.86 g/mol |
| Melting Point | 134℃(dec.) |
| Sensitive | Hygroscopic, Air Sensitive, Heat Sensitive |
| Storage Temp. | Cool & Dry Place (2~8℃) |
| COA & MSDS | Available |
| Origin | Shanghai, China |
| Brand | Ruifu Chemical |
| Items | Specifications | Results |
| Appearance | White to Off-White Crystalline Powder | Complies |
| Identification A | Infrared Absorption | Complies |
| Identification B | Ultraviolet Absorption | Complies |
| Identification C | Tests for Chloride | Complies |
| pH | 4.5~6.0 | 5.42 |
| Loss on Drying | <0.75% | 0.32% |
| Residue on Ignition | <0.10% | 0.05% |
| Ranitidine Bis-Compound | <0.30% | 0.03% |
| Any Other Single Impurity | <0.10% | 0.04% |
| Total Impurities | <0.50% | 0.16% |
| Assay / Analysis Method | 97.5~102.0% (Calculated on the Dried Basis) | 99.70% |
| Conclusion | The product has been tested and complies with the USP35 specifications | |
| Shelf life | 24 Months if Stored Properly | |
Package: Fluorinated Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Keep the container tightly closed and store in a cool, dry (2~8℃) and well-ventilated warehouse away from incompatible substances. Keep away from strong, direct light and moisture, avoid fire and heat. Incompatible with oxidizing agents.
Shipping: Deliver to worldwide by air, by FedEx / DHL Express. Provide fast and reliable delivery.
Ranitidine Hydrochloride
C13H22N4O3S·HCl 350.87
1,1-Ethenediamine, N-[2-[[[5-[(dimethylamino)methyl]-2-furanyl]-methyl]thio]ethyl]-N¢-methyl-2-nitro-, monohydrochloride.
N-[2-[[[5-[(Dimethylamino)methyl]-2-furanyl]methyl]thio]ethyl]-N¢-methyl-2-nitro-1,1-ethenediamine, hydrochloride [66357-59-3].
Ranitidine Hydrochloride contains not less than 97.5 percent and not more than 102.0 percent of C13H22N4O3S·HCl, calculated on the dried basis.
Packaging and storage-Preserve in tight, light-resistant containers.
USP Reference standards <11>-
USP Ranitidine Hydrochloride RS
USP Ranitidine Resolution Mixture RS
It is a mixture of ranitidine hydrochloride and four related impurities: ranitidine-N-oxide, ranitidine complex nitroacetamide, ranitidine diamine hemifumarate, and ranitidine amino alcohol hemifumarate.
Ranitidine-N-oxide: N,N-dimethyl[5-[[[2-[[1-(methylamino)-2-nitroethenyl]amino]ethyl]sulphanyl]methyl]furan-2-yl]methanamine N-oxide.
Ranitidine complex nitroacetamide: N-[2-[[[5-[(dimethylamino)methyl]furan-2-yl]methyl]sulphanyl]ethyl]-2-nitroacetamide.
Ranitidine diamine hemifumarate (related compound A): 5-[[(2-aminoethyl)thio]methyl]-N,N-dimethyl-2-furanmethanamine, hemifumarate salt.
Ranitidine amino alcohol hemifumarate: [5-[(dimethylamino)methyl]furan-2-yl]methanol.
Identification-
A: Infrared Absorption <197M>.
B: Ultraviolet Absorption <197U>-
Solution: 10 µg per mL.
Medium: water.
Absorptivities at 229 nm and 315 nm, calculated on the dried basis, do not differ by more than 3.0%.
C: A solution of it meets the requirements of the tests for Chloride <191>.
pH <791>: between 4.5 and 6.0, in a solution (1 in 100).
Loss on drying <731>-Dry it in vacuum at 60 for 3 hours: it loses not more than 0.75% of its weight.
Residue on ignition <281>: not more than 0.1%.
Chromatographic purity-
Diluent, Mobile phase, Resolution solution, and Chromatographic system-Proceed as directed in the Assay.
Standard solution-Prepare as directed for Standard preparation in the Assay.
Test solution-Prepare as directed for Assay preparation in the Assay.
Procedure-Separately inject equal volumes (about 10 µL) of the Standard solution and the Test solution into the chromatograph, record the chromatograms, and identify the ranitidine peak and the peaks due to impurities and degradation products listed in the table below.
Name Relative Retention Time
Ranitidine simple nitroacetamide1 0.14
Ranitidine oxime2 0.21
Ranitidine amino alcohol3 0.45
Ranitidine diamine4 0.57
Ranitidine S-oxide5 0.64
Ranitidine N-oxide6 0.72
Ranitidine complex nitroacetamide7 0.84
Ranitidine formaldehyde adduct8 1.36
Ranitidine bis-compound9 1.75
1 N-Methyl-2-nitroacetamide.
2 3-(Methylamino)-5,6-dihydro-2H-1,4-thiazin-2-one oxime.
3 {5-[(Dimethylamino)methyl]furan-2-yl}methanol.
4 5-{[(2-Aminoethyl)thio]methyl}-N,N-dimethyl-2-furanmethanamine (ranitidine related compound A).
5 N-{2-[({5-[(Dimethylamino)methyl]-2-furanyl}methyl)sulfinyl]ethyl}-N¢-methyl-2-nitro-1,1-ethenediamine (ranitidine related compound C).
6 N,N-Dimethyl(5-{[(2-{[1-(methylamino)-2-nitroethenyl]amino}ethyl)
sulphanyl]methyl}furan-2-yl)methanamine N-oxide.
7 N-{2-[({5-[(Dimethylamino)methyl]furan-2-yl}methyl)sulphanyl]ethyl}-2-nitroacetamide.
8 2,2¢-Methylenebis(N-{2-[({5-[(dimethylamino)methyl]furan-2-yl}methyl)sulphanyl]ethyl}-N¢-methyl-2-nitroethene-1,1-diamine).
9 N,N¢-bis{2-[({5-[(Dimethylamino)methyl]-2-furanyl}methyl)thio]ethyl}-2-nitro-1,1-ethenediamine (ranitidine related compound B).
Measure the responses for the major peaks, and calculate the percentage of each impurity in the portion of Ranitidine Hydrochloride taken by the formula:
100CV/W(ri / rS)
in which C is the concentration, in mg per mL, of ranitidine hydrochloride in the Standard solution; V is the volume, in mL, of the Test solution; W is the weight, in mg, of Ranitidine Hydrochloride taken to prepare the Test solution; ri is the peak response for each impurity obtained from the Test solution; and rS is the ranitidine peak response obtained from the Standard solution: not more than 0.3% of ranitidine bis-compound is found, not more than 0.1% of any other single impurity is found, and not more than 0.5% of total impurities is found. The reporting level for impurities is 0.05%.
Assay-
Phosphate buffer-Place approximately 1900 mL of water in a 2.0-L volumetric flask, accurately add 6.8 mL of phosphoric acid, and mix. Accurately add 8.6 mL of 50% sodium hydroxide solution, and dilute with water to volume. If necessary, adjust with 50% sodium hydroxide solution or phosphoric acid to a pH of 7.1, and filter.
Solution A-Prepare a mixture of Phosphate buffer and acetonitrile (98:2).
Solution B-Prepare a mixture of Phosphate buffer and acetonitrile (78:22).
Mobile phase-Use variable mixtures of Solution A and Solution B as directed for Chromatographic system. Make adjustments if necessary (see System Suitability under Chromatography 621).
Diluent-Use Solution A.
Standard preparation-Dissolve an accurately weighed quantity of USP Ranitidine Hydrochloride RS in Diluent to obtain a solution having a known concentration of about 0.125 mg of ranitidine hydrochloride per mL.
Resolution solution-Transfer about 1.3 mg of USP Ranitidine Resolution Mixture RS to a 10-mL volumetric flask, and dissolve in and dilute with Diluent to volume. [note—USP Ranitidine Resolution Mixture RS contains ranitidine hydrochloride and four related impurities: ranitidine amino alcohol hemifumarate, ranitidine diamine hemifumarate, ranitidine N-oxide, and ranitidine complex nitroacetamide. ]
Assay preparation-Transfer about 25 mg of Ranitidine Hydrochloride, accurately weighed, to a 200-mL volumetric flask. Dissolve in and dilute with Diluent to volume, and mix.
Chromatographic system (see Chromatography <621>)-The liquid chromatograph is equipped with a 230-nm detector and a 4.6-mm × 10-cm column containing 3.5-µm packing L1 that is stable from pH 1 to 12. The flow rate is about 1.5 mL per minute. The column temperature is maintained at 35. The chromatograph is programmed as follows.
Time (minutes) Solution A (%) Solution B (%) Elution
0-10 100®0 0®100 linear gradient
10-15 0 100 isocratic
15-16 0®100 100®0 linear gradient
16-20 100 0 re-equilibrationChromatograph the Resolution solution, and identify the peaks using the table of impurities and degradation products (found above): the resolution, R, between the peaks for ranitidine N-oxide and ranitidine complex nitroacetamide is not less than 1.5. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the relative standard deviation for replicate injections is not more than 1.0%.
Procedure-Separately inject equal volumes (about 10 µL) of the Standard preparation and the Assay preparation into the chromatograph, record the chromatograms, and measure the areas for the major peaks. Calculate the percentage of C13H22N4O3S·HCl in the portion of Ranitidine Hydrochloride taken by the formula:
100(CS / CU)(rU / rS)
in which CS and CU are the concentrations, in mg per mL, of ranitidine hydrochloride in the Standard preparation and the Assay preparation, respectively; and rU and rS are the peak responses obtained from the Assay preparation and the Standard preparation, respectively.
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc.
Advantages? Superior quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
Safety Description S22 - Do not breathe dust.
S24/25 - Avoid contact with skin and eyes.
WGK Germany 2
RTECS KM6557000
Ranitidine Hydrochloride (CAS: 66357-59-3) is a kind of histamine H2-receptor antagonist which inhibits gastric acid secretion. Antiulcerative. Since its listing in 1981, Ranitidine Hydrochloride has been widely used in nearly one hundred countries in the world, including China. It is clinically used for the treatment of duodenal ulcer, reflux esophagitis and Zollinger-Ellison syndrome. It is also used for the prevention of gastrointestinal bleeding caused by stress ulcer and recurrent hemorrhage of peptic ulcer. In recent decade, through the combination of ranitidine and other drugs, it has found that it has high efficiency and remarkable features in the treatment of Helicobacter pylori-positive duodenal ulcer, urticaria and post-cerebral hemorrhage stress ulcer with better efficacy than other similar drugs. Owing to its rapid effect, good potency and low price, ranitidine hydrochloride plays an important role in the anti-ulcer drug market today. Therefore, strict quality control plays important roles in guiding patients with reasonable, safe medication. Ranitidine is also used alongside and other antihistamines for the treatment of skin conditions such as. Ranitidine HCl is marketed under the brand name Zinetac or Zantac. As a histamine H2 receptor blocker, which can inhibit the secretion of basic gastric acid and gastric acid after stimulation, as well as the secretion of pepsin.Its acid inhibition is 5~8 times stronger than Cimetidine.
For the treatment of duodenal ulcer, gastric ulcer, reflux esophagitis, Zollinger-Ellison syndrome and other high acid secretion disorders.
Common reactions are: nausea, rash, constipation, fatigue, headache, dizziness and so on.
Light adverse reactions on the renal function, gonadal function and central nervous system.
A small number of patients get mild liver damage after taking the drug with the symptoms disappearing after stopping, liver function returned to normal.
Ranitidine Hydrochloride, an H2-receptor antagonist, can cause contact dermatitis within the pharmaceutical industry and in health care workers, or may induce systemic drug reactions in patients.
-
Ranitidine Hydrochloride CAS 66357-59-3 Assay 9...
-
Vonoprazan Fumarate (TAK-438) CAS 1260141-27-2 ...
-
Acyclovir CAS 59277-89-3 Assay 98.0-101.0% (HPL...
-
Azilsartan CAS 147403-03-0 Purity >99.5% (HPLC)...
-
Azithromycin Dihydrate CAS 117772-70-0 Assay 94...
-
Baloxavir Marboxil CAS 1985606-14-1 API Factory...
-
Betamethasone CAS 378-44-9 Purity 97.0%~103.0% ...
-
Bicalutamide CAS 90357-06-5 API Factory High Qu...
-
Bortezomib CAS 179324-69-7 Purity ≥99.0% (HPLC)...
-
Brexpiprazole CAS 913611-97-9 Purity >99.0% (HP...
-
Candesartan Cilexetil CAS 145040-37-5 Purity >9...
-
Captopril CAS 62571-86-2 API Factory USP High Q...
-
CAS 177036-94-1 Purity >99.0% (HPLC) API Factor...
-
Caspofungin Acetate Cancidas CAS 179463-17-3 AP...
-
Cefotaxime Sodium Salt CAS 64485-93-4 Assay ≥91...
-
Paclitaxel (Taxol) CAS 33069-62-4 Assay (HPLC) ...