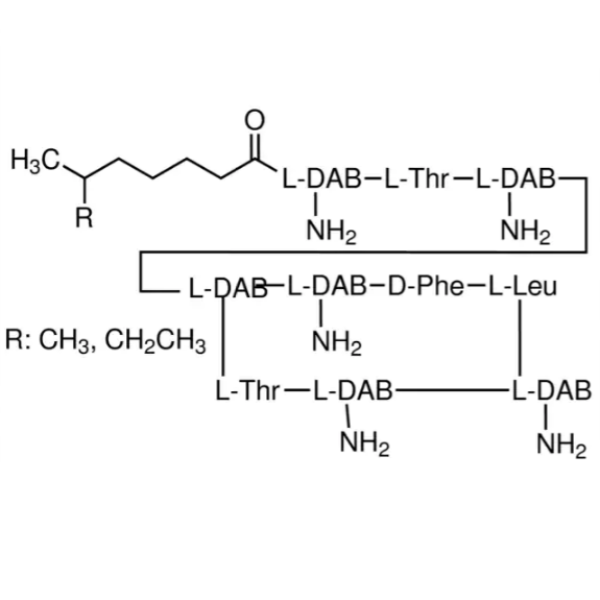
Polymyxin B Sulfate CAS 1405-20-5 Assay Microbiological ≥6500 IU/mg Factory
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer and supplier of Polymyxin B Sulfate (CAS: 1405-20-5) with high quality. We can provide COA, worldwide delivery, small and bulk quantities available. If you are interested in Polymyxin B Sulfate, Please contact: alvin@ruifuchem.com
| Chemical Name | Polymyxin B Sulfate |
| Synonyms | Polymyxin B Sulfate Salt; Aerosporin; Mastimyxin; PXNS; PMB; Poly-RX |
| Stock Status | In Stock, Production Capacity 10 Tons per Year |
| CAS Number | 1405-20-5 |
| Molecular Formula | C55H96N16O13·2H2SO4 |
| Molecular Weight | 1301.56 |
| Melting Point | 217.0~220.0℃(dec.) |
| Sensitive | Hygroscopic. Sensitive to Light and Heat |
| Water Solubility | Soluble in Water, 50 mg/mL. Slightly Soluble in Ethanol |
| COA & MSDS | Available |
| Sample | Available |
| Brand | Ruifu Chemical |
| Items | Specifications | Results |
| Appearance | White or Almost With Crystalline Powder | Conforms |
|
Identification A. The retention times of the major peaks from the sample solution correspond to those of the standard solution, as obtained in the test for composition of Polymyxins. |
Conforms |
|
| PH (2%) |
5.0~7.0 |
5.7 |
| PH (0.5%) |
5.0~7.5 |
5.5 |
| Sulphated Ash |
≤0.75% |
|
| Specific Rotation [α]20/D |
-78.0°~-90.0°(C=2, H2O) (Calcd. on Anh. Substance) |
-85.0° |
| Sulphate |
15.5~17.5% |
16.65% |
| Loss on Drying |
≤6.00% |
3.0% |
| Residue on Ignition |
≤0.10% |
0.02% |
| Heavy Metals |
≤20ppm |
≤20ppm |
| Phenylalanine |
9.0~12.0% |
9.8% |
| Total Viabel Aerobic Count |
≤10cfu/g |
≤10cfu/g |
| Related Substances, HPLC |
|
|
| Individual Impurity |
≤3.0% |
2.723% |
| Total Impurities |
≤17.0% |
16.223% |
|
Assay HPLC (ODB) |
Sum of Polymyxins B1,B2,B3 and B1-1: ≥80.0% |
86.6% |
|
Polymyxin B3: ≤ 6.0% |
3.8% |
|
|
Polymyxin B1-1: ≤15.0% |
8.5% |
|
| Assay, Microbiological (as is) |
≥6500lU/mg |
8255IU/mg |
|
Particle Size |
≥99.0% ≤30um by vol |
Conforms |
|
≥90.0% ≤25um by vol |
||
|
≥75.0%≤20um by vol |
||
|
≥35.0%≤10um by vol |
||
| Conclusion |
The product complies USP42 |
|
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Light sensitive. Keep the container tightly closed and store in a cool, dry (2~8℃) and well-ventilated warehouse away from incompatible substances. Protect from light and moisture. Incompatible with strong oxidizing agents.
Shipping: Deliver to worldwide by air, by FedEx / DHL Express. Provide fast and reliable delivery.
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc.
Advantages? Superior quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
Hazard Symbols Xn - Harmful
Risk Codes 22 - Harmful if swallowed
Safety Description S22 - Do not breathe dust.
S24/25 - Avoid contact with skin and eyes.
UN IDs 3249
WGK Germany 3
RTECS TR1150000
FLUKA BRAND F CODES 3-8-10
HS Code 2941909099
Hazard Class 6.1(b)
Packing Group III
Polymyxin is a group of polypeptide antibiotics produced by Bacillus polymyxa (bacilluspolymyxa). Polymyxin B and E are used for medicinal purposes, and their sulfates are commonly used. The antibacterial spectrum and clinical application of Polymyxin B Sulfate are similar to those of polymyxin E. It has inhibitory or bactericidal effects on gram-negative bacilli, such as Escherichia coli, Pseudomonas aeruginosa, paracilli, Klebsiella pneumoniae, acidophilus, pertussis and Shigella.
Polymyxin Sulfate B is similar to polymyxin e, and has inhibitory or bactericidal effects on gram-negative bacilli, such as e. coli, pseudomonas aeruginosa, paracilli, Klebsiella pneumoniae, acidophilus, bacilli pertussis and dysentery bacilli. It is mainly used in the wound, urinary tract, eye, ear, trachea and other parts of infection caused by Pseudomonas aeruginosa and other Pseudomonas. It can also be used for sepsis and peritonitis. And severe infections caused by aminoglycosides, third-generation cephalosporin-resistant bacteria, Pseudomonas aeruginosa or other sensitive bacteria, such as bacteremia, endocarditis, pneumonia, post-burn infection, etc.
1. Intravenous drip: adults and children with normal renal function 1.5~2.5mg per kilogram of body weight (generally not more than 2.5mg per kilogram of body weight) for 1 day, divided into 2 times, drip once every 12 hours. Every 50mg of this product is diluted with 500ml of 5% glucose solution and then dripped. Infants with normal renal function can tolerate 4mg per kilogram of body weight for 1 day. 2. Intramuscular injection: adults and children: 2.5~3mg per kilogram of body weight on the 1st, given in batches, once every 4~6 hours. Infants can reach 4mg per kilogram of body weight per day and newborns can reach 4.5mg per kilogram of body weight. 3. Intrathecal injection (for Pseudomonas aeruginosa meningitis): 5mg per milliliter of liquid medicine prepared with sodium chloride injection. Adults and children over 2 years old, 5mg daily, after 3-4 days of application, change to once every other day, at least 2 weeks, until the cerebrospinal fluid culture is negative, and the sugar content is normal. For children under 2 years old, use 2mg once a day for 3-4 consecutive days (or 2.5mg once every other day), and then use 2.5mg once every other day until the test is normal. 4. The concentration of eye drops is 1~2.5mg per ml.
Avoid combining with drugs that damage the kidneys, skeletal muscle relaxants, aminoglycoside antibiotics, anesthetics with obvious muscle relaxation effects (such as enflurane), etc., and quinine, magnesium, etc. cannot be used intravenously at the same time. Polymyxin B sulfate is combined with sulfonamides, rifampicin, semi-synthetic penicillin, etc., for the treatment of severe drug-resistant gram-negative bacterial infections, and the effect is better than that of single application.
1, Pregnant and lactating women, children, and patients with severe renal insufficiency are more common. 2. This product is highly toxic and has poor curative effect on deep tissue infection. It is not the first choice for any infection. 3. Intravenous injection may cause respiratory depression, which is generally not used. At least half of the daily dose requires intravenous drip, not a quick bolus at once, so as to avoid extensive neuromuscular block. 4. It should not be combined with other drugs with nephrotoxicity or neuromuscular blockade to avoid accidents.
Biochemical research. Polymyxin B is an anti-gram-negative bacilli polypeptide antibiotic, which can change the membrane structure to leak small molecules and inhibit the growth of gram-negative bacteria. It has a bactericidal effect on Escherichia coli. Lipid A moieties bound to bacterial lipopolysaccharides. Small pores are induced in the outer skin cell membrane. Mode of action: binds and interferes with the permeability of the cytoplasmic membrane. Antibacterial spectrum: Gram-negative bacteria.
Polymyxin B, Sulfate.
Polymyxin B Sulfate [1405-20-5].
» Polymyxin B Sulfate is the sulfate salt of a kind of polymyxin, a substance produced by the growth of Bacillus polymyxa (Prazmowski) Migula (Fam. Bacillaceae), or a mixture of two or more such salts. It has a potency of not less than 6000 Polymyxin B Units per mg, calculated on the dried basis.
Packaging and storage-Preserve in tight, light-resistant containers.
Labeling-Where packaged for prescription compounding, the label states the number of Polymyxin B Units in the container and per milligram, that it is not intended for manufacturing use, that it is not sterile, and that its potency cannot be assured for longer than 60 days after opening. Where it is intended for use in preparing injectable or other sterile dosage forms, the label states that it is sterile or must be subjected to further processing during the preparation of injectable or other sterile dosage forms.
USP Reference standards <11>-
USP Polymyxin B Sulfate RS
Identification-
A: Liquid Chromatographic Identification Test-
Mobile phase-Prepare a mixture of 0.1 M tribasic sodium phosphate and acetonitrile (77:23), and adjust with phosphoric acid to a pH of 3.0. Make adjustments if necessary (see System Suitability under Chromatography <621>.
Standard solution-Prepare a solution of USP Polymyxin B Sulfate RS in Mobile phase having a concentration of about 3.5 mg per mL. Protect this solution from light.
Test solution-Prepare a solution of Polymyxin B Sulfate in Mobile phase having a concentration of about 3.5 mg per mL. Protect this solution from light.
Chromatographic system (see Chromatography <621>)-The liquid chromatograph is equipped with a 212-nm detector and a 4.6-mm × 25-cm column that contains 5-µm packing L1. The flow rate is about 1 mL per minute.
Procedure-Separately inject equal volumes (about 10 µL) of the Standard solution and the Test solution into the chromatograph, and record the chromatograms. The chromatogram obtained from the Test solution corresponds qualitatively to that obtained from the Standard solution, exhibiting a major peak corresponding to polymyxin B1 and peaks at relative retention times of about 0.5 (polymyxin B2) and 0.6 (polymyxin B3).
B: Dissolve 2 mg in 5 mL of water, add 5 mL of 2.5 N sodium hydroxide, mix, and add 5 drops of cupric sulfate solution (1 in 100), shaking after the addition of each drop: a reddish violet color is produced.
C: A solution (1 in 20) meets the requirements of the tests for Sulfate <191>.
pH <791>: between 5.0 and 7.5, in a solution containing 5 mg per mL.
Loss on drying <731>-Dry about 100 mg, accurately weighed, in a capillary-stoppered bottle in vacuum at 60℃ for 3 hours: it loses not more than 7.0% of its weight.
Content of phenylalanine-Transfer about 0.375 g of Polymyxin B Sulfate, accurately weighed, to a 100-mL volumetric flask, dissolve in and dilute with 0.1 N hydrochloric acid to volume, and mix. Measure the absorbances of this solution at the maxima at about 264 nm (A264), 258 nm (A258), and 252 nm (A252), and the absorbances at 280 nm (A280) and 300 nm (A300). Calculate the percentage of phenylalanine in the portion of Polymyxin B Sulfate taken by the formula:
(9.4787/W)(A258 – 0.5A252 + 0.5A264 – 1.84A280 + 0.8A300)
in which W is the weight, in g, of Polymyxin B Sulfate taken: it contains between 9% and 12% of phenylalanine, calculated on the dried basis.
Other requirements- If for prescription compounding, it meets the requirements for Residue on ignition under Polymyxin B for Injection. Where the label states that Polymyxin B Sulfate is sterile, it meets the requirements for Sterility Tests <71> and, where intended for injectable dosage forms, for Pyrogen under Polymyxin B for Injection. Where the label states that Polymyxin B Sulfate must be subjected to further processing during the preparation of injectable dosage forms, it meets the requirements for Pyrogen under Polymyxin B for Injection.
Assay-Proceed with Polymyxin B Sulfate as directed under Antibiotics-Microbial Assays <81>.