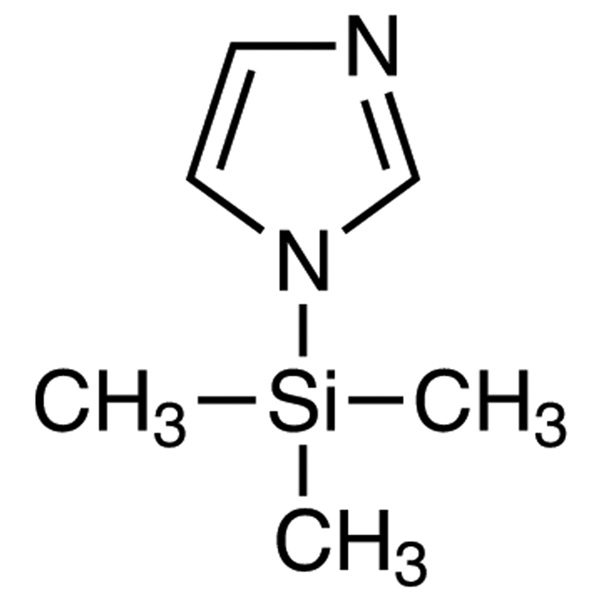
N-(Trimethylsilyl)imidazole (TSIM) CAS 18156-74-6 Purity >99.0% (GC) Factory Main Product
Manufacturer Supply, High Purity, Commercial Production
Chemical Name: N-(Trimethylsilyl)imidazole (TSIM)
CAS: 18156-74-6
| Chemical Name | N-(Trimethylsilyl)imidazole |
| Synonyms | TSIM; 1-(Trimethylsilyl)imidazole; TMS-Imidazole; N-Trimethylsilylimidazole; 1-(Trimethylsilyl)-1H-Imidazole |
| CAS Number | 18156-74-6 |
| CAT Number | RF-PI978 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C6H12N2Si |
| Molecular Weight | 140.26 |
| Melting Point | -42℃ |
| Boiling Point | 93.0~94.0℃/14 mmHg (lit.) |
| Specific Gravity (20/20) | 0.957 g/mL |
| Refractive Index | n20/D 1.4751~1.4780 (lit.) |
| Solubility | Soluble in Acetonitrile, Acetone, Pyridine |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | Colorless Clear Liquid |
| Purity / Analysis Method | >99.0% (GC) |
| Total Impurities | <1.0% |
| Properties | Deliquescence Easily |
| Test Standard | Enterprise Standard |
| Usage | Silanizing Protectant; Silylation Reagents; for Gas Chromatography |
Package: Bottle, 25kg/Barrel, or 180kg/Drum, or according to customer's requirement.
Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture.


N-(Trimethylsilyl)imidazole (TSIM) (CAS: 18156-74-6) is a general silylating agent, particularly for alcohols. An intermediate for the synthesis of imidazole derivatives. Silylating reagent for the protection of hydroxyl groups in the presence of amine functionalities. N-(Trimethylsilyl)imidazole is a derivatisation agent used in gas chromatography/mass spectrometry applications. N-(Trimethylsilyl)imidazole is a silylating agent for alcohols and 1,3-dicarbonyl compounds; reaction with esters to give imidazolides; preparation of O-trimethylsilyl monothioacetals; aromatization of the A-ring of steroids. It participates in the reactions of Hydroxyl Silylation Reactions, Silyl Aminal Formation Reactions, Nitrogen Silylation Reactions, Acyl Imidazole Formation, Michael Addition Reactions, Substitution Reactions, Phosphoroimidazolidate Formation, and other uses.
-
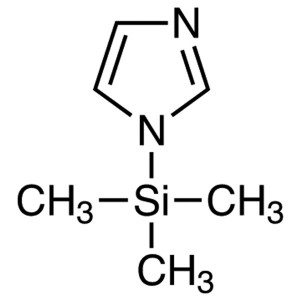
N-(Trimethylsilyl)imidazole (TSIM) CAS 18156-74...
-
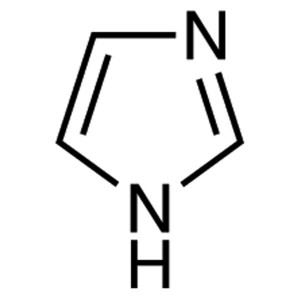
Imidazole CAS 288-32-4 Purity ≥99.5% (GC) Facto...
-
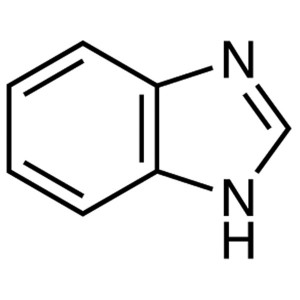
Benzimidazole CAS 51-17-2 Purity ≥99.5% (HPLC) ...
-
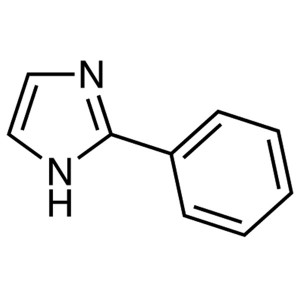
2-Phenylimidazole CAS 670-96-2 Purity >99.0% (G...
-
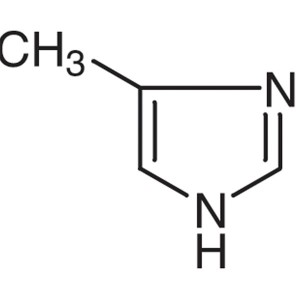
4-Methylimidazole CAS 822-36-6 Purity ≥99.5% (G...
-
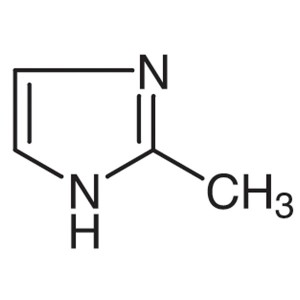
2-Methylimidazole CAS 693-98-1 Purity >99.5% (G...
-
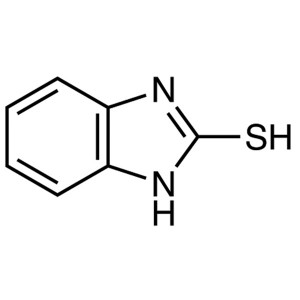
2-Mercaptobenzimidazole CAS 583-39-1 Purity ≥99...
-
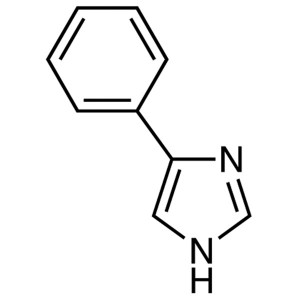
4-Phenylimidazole CAS 670-95-1 Purity ≥99.0% (H...
-
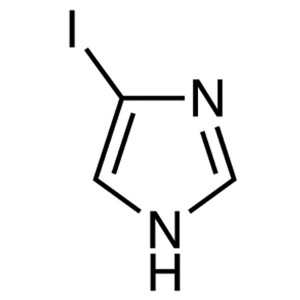
4-Iodoimidazole CAS 71759-89-2 Purity ≥99.5% (H...
-
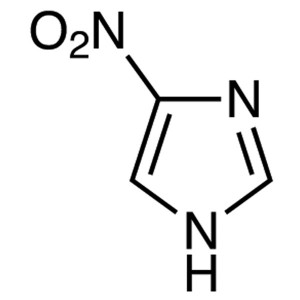
4-Nitroimidazole CAS 3034-38-6 Purity ≥99.0% (G...
-
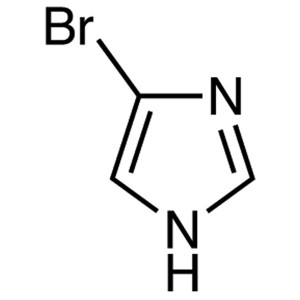
4-Bromoimidazole CAS 2302-25-2 Purity >99.0% (G...
-
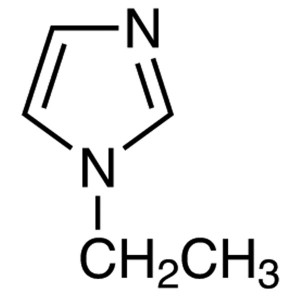
1-Ethylimidazole CAS 7098-07-9 Purity >98.0% (G...
-
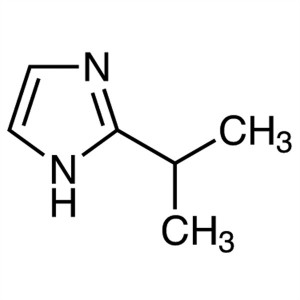
2-Isopropylimidazole CAS 36947-68-9 Purity >99....