Low price for Canagliflozin - Levetiracetam LEV CAS 102767-28-2 API USP EP Standard High Purity – Ruifu
Low price for Canagliflozin - Levetiracetam LEV CAS 102767-28-2 API USP EP Standard High Purity – Ruifu Detail:
Manufacturer Supply with High Purity and Stable Quality
Chemical Name: Levetiracetam
CAS: 102767-28-2
API USP/EP Standard High Purity
A Third-generation Antiepileptic Drug
API High Quality, Commercial Production
| Chemical Name | Levetiracetam |
| Synonyms | (S)-2-(2-Oxo-1-pyrrolidinyl)butyramide; UCB-L059 |
| CAS Number | 102767-28-2 |
| CAT Number | RF-API61 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C8H14N2O2 |
| Molecular Weight | 170.21 |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | White or Almost White Crystals Powder |
| Identification IR | The Spectrum abtained from sample consists with that obtained from reference substance |
| Appearance of Solution | Clear and not more intensely coloured than BY6 |
| Enantiomeric Purity ImpurityD | ≤0.80% |
| Water | ≤0.50% |
| Sulfated Ash | ≤0.10% |
| Heavy Metals | ≤0.001% |
| Related Substances | |
| Impurity A | ≤0.30% |
| Any Unspecified Impurity | ≤0.05% |
| Total Unspecified Impurities | ≤0.10% |
| Total Impurities | ≤0.40% |
| Impurity F | ≤0.10% |
| Residual Solvents | |
| Benzene | ≤2ppm |
| Dichloromethane | ≤600ppm |
| Ethyl Acetate | ≤5000ppm |
| Acetone | ≤5000ppm |
| Assay | 98.0%~102.0% |
| Test Standard | United States Pharmacopoeia (USP) Standard; European Pharmacopeia (EP) Standard |
| Usage | Active Pharmaceutical Ingredient (API) |
Package: Bottle, Aluminum foil bag, Cardboard drum, 25kg/Drum, or according to customer’s requirement.
Storage Condition: Store in sealed containers at cool and dry place; Protect from light, moisture and pest infestation.


Levetiracetam, a derivative of pilacetam, is a novel third-generation antiepileptic drug approved by the US FDA in 1999. It was initially used for the adjunctive treatment of partial seizures in adults. In 2005, Levetiracetam was approved in oral tablets and solutions for the adjunctive treatment of partial seizures in children aged 4 years and older. It is mainly used for the additive treatment of partial seizures in adults and children over 4 years old, and can also be used only for partial seizures and systemic seizures in adults. It also has certain curative effect on myoclonic epilepsy in teenagers, refractory epilepsy, absent epilepsy in children and persistent epilepsy.LEV has been reported to improve cognitive function in patients with epilepsy, and can also be used for myoclonus caused by other causes (such as encephalitis and cerebral hypoxia).
Levetiracetam is a relatively unique anticonvulsant that is typically used in combination with other antiepileptic medications for partial onset seizures. Levetiracetam is an antiepileptic that displays distinctive properties from conventional antiepileptic drugs. Displays potent seizure protection in animal models of chronic epilepsy but lacks activity in acute seizure models. Binds synaptic vesicle protein 2A (SV2A) and inhibits Na+-dependent Cl-/HCO3- exchange.
Product detail pictures:
Related Product Guide:
We've got a really efficient group to deal with inquiries from prospects. Our purpose is "100% customer fulfillment by our product excellent, price & our group service" and enjoy a superb track record amid clientele. With many factories, we can easily deliver a wide selection of Low price for Canagliflozin - Levetiracetam LEV CAS 102767-28-2 API USP EP Standard High Purity – Ruifu , The product will supply to all over the world, such as: USA, Indonesia, Poland, We also have good cooperation relationships with many good manufacturers so that we can provide almost all of auto parts and after-sales service with high quality standard,lower price level and warmly service to meet demands of customers from different fields and different area.
-
Good Wholesale Vendors Guanfacine Hydrochloride...
-
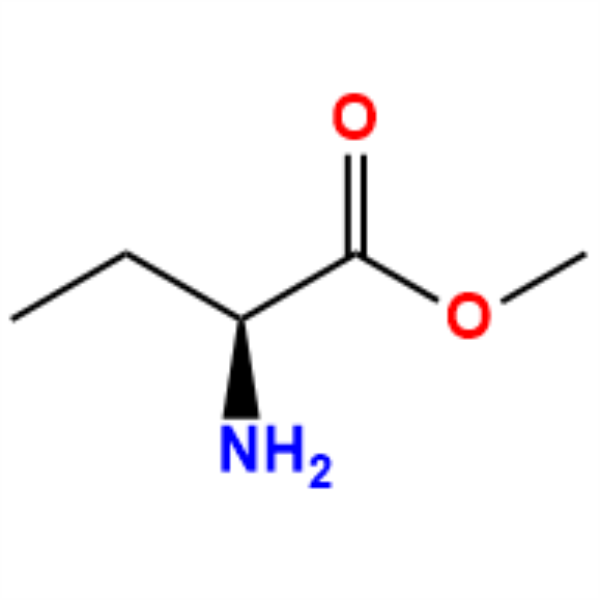
PriceList for R-Glycidyl Tosylate - (S)-Methyl...
-
Bottom price 4-Guanidinobenzoic Acid Hydrochlor...
-
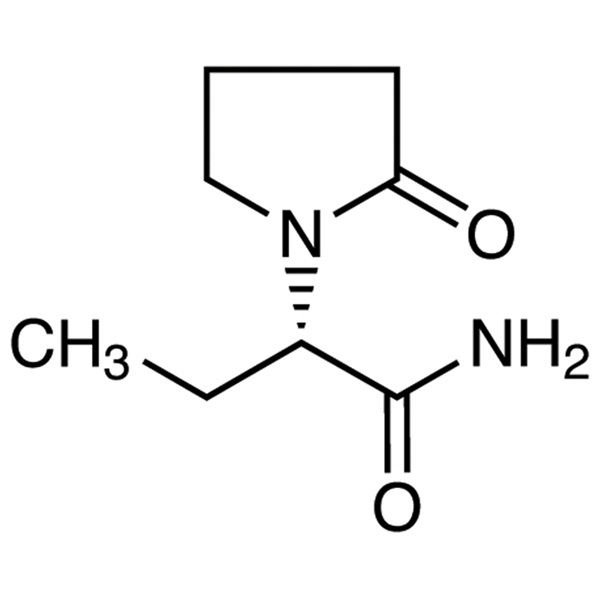
Massive Selection for Levodopa - Teneligliptin...
-
Hot Sale for Uridine 5-Monophosphate Disodium S...
-
Manufacturing Companies for ATS-9 - N-(5-Amino...
Production management mechanism is completed, quality is guaranteed, high credibility and service let the cooperation is easy, perfect!
