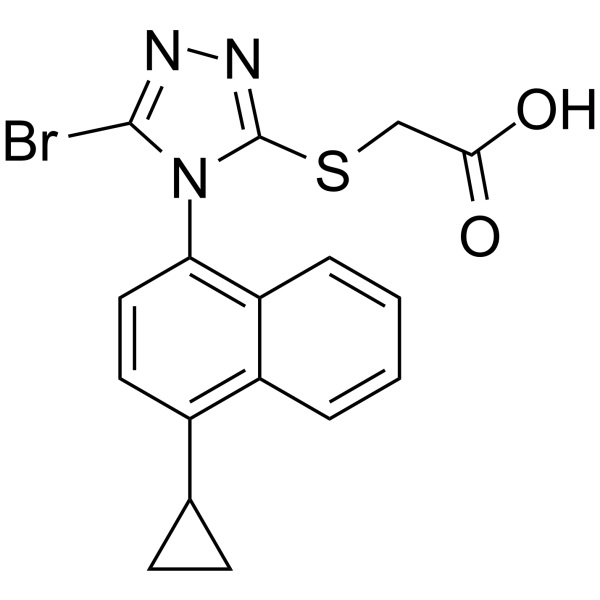
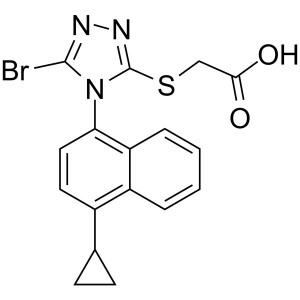
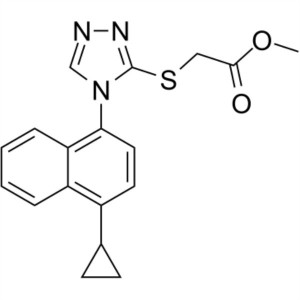
Lesinurad (RDEA 594) CAS 878672-00-5 Purity >99.5% (HPLC) Assay 98.0~102.0%
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer of Lesinurad (RDEA 594) (CAS: 878672-00-5) with high quality. Ruifu Chemical can provide worldwide delivery, competitive price, excellent service, small and bulk quantities available. Purchase Lesinurad and Intermediates, Please contact: alvin@ruifuchem.com
| Chemical Name | Lesinurad |
| Synonyms | 2-[[5-Bromo-4-(4-Cyclopropylnaphthalen-1-yl)-4H-1,2,4-Triazol-3-yl]thio]acetic Acid; 2-[[5-Bromo-4-(4-Cyclopropyl-1-Naphthalenyl)-4H-1,2,4-Triazol-3-yl]thio]-Acetic Acid; RDEA 594; RDEA-594; RDEA594; Zurampic |
| Stock Status | In Stock, Commercial Production |
| CAS Number | 878672-00-5 |
| Molecular Formula | C17H14BrN3O2S |
| Molecular Weight | 404.28 g/mol |
| Melting Point | >171℃(dec.) |
| Density | 1.72±0.10 g/cm3 |
| COA & MSDS | Available |
| Origin | Shanghai, China |
| Brand | Ruifu Chemical |
| Items | Inspection Standards | Results |
| Appearance | White to Off-White Powder | Off-White Powder |
| Identification | The retention time of major peak of the sample solution corresponds to the reference standard | Complies |
| Water by Karl Fischer | <0.50% | 0.14% |
| Residue on Ignition | <0.10% | 0.03% |
| Heavy Metals (Pb) | ≤10ppm | <10ppm |
| Related Substances (HPLC) | ||
| The Largest Single impurity | <0.20% | 0.07% |
| Total Impurities | <1.00% | 0.24% |
| Purity / Analysis Method | >99.5% (HPLC Area) | 99.76% |
| Assay | 98.0~102.0% | 99.6% |
| Infrared Spectrum | Conforms to Structure | Complies |
| NMR Spectrum | Conforms to Structure | Complies |
| Conclusion | The product has been tested and complies with the given specifications | |
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Keep the container tightly closed and store in a cool, dry (2~8℃) and well-ventilated warehouse away from incompatible substances. Protect from light and moisture.
Shipping: Deliver to worldwide by air, by FedEx / DHL Express. Provide fast and reliable delivery.
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc.
Advantages? Superior quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
Lesinurad (RDEA 594) (CAS: 878672-00-5) was approved by the U.S. Food and Drug Administration (FDA) on December 22, 2015, and then approved by the European Medicines Agency (EMA) on February 18, 2016. The compound was earlier developed by Ardea. AstraZeneca acquired the company in 2012, which in turn acquired the product. The drug's trade name is Zurampic® in the United States and the European Union. Lesinurad is the world's first approved urate reuptake transporter (URAT1) inhibitor, combined with xanthine oxidase inhibitors to treat hyperuricemia-related gout.
Lesinurad, trade name Zurampic. Lesinurad in combination with xanthine oxidase inhibitor (XOI) for hyperuricemic gout syndrome. Its mechanism of action is to help the kidneys excrete uric acid, and it works by inhibiting the function of transporters involved in uric acid reabsorption in the kidneys.
Lesinurad inhibits the uric acid transport activity (IC50=3.36 μM) of hURAT1 (human URAT1), which is more than 20 times the inhibitory effect on rURAT1 (rat URAT1) (IC50=74.84 μM). Lesinurad inhibit hURAT1 through the interaction of the key amino acid residue Phe365.