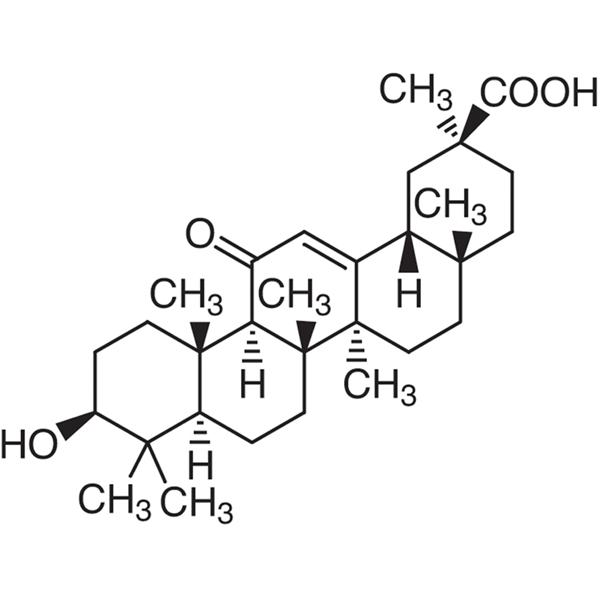
Glycyrrhetic Acid (Enoxolone) CAS 471-53-4 Assay 98.0~101.0% (Potentiometry)
Ruifu Chemical is the leading manufacturer of Glycyrrhetic Acid (18β-Glycyrrhetinic Acid; Enoxolone) (CAS: 471-53-4) with high quality. Ruifu Chemical can provide worldwide delivery, competitive price, excellent service, small and bulk quantities available. Purchase Glycyrrhetic Acid, Please contact: alvin@ruifuchem.com
| Chemical Name | Glycyrrhetic Acid |
| Synonyms | 18β-Glycyrrhetinic Acid; 18-beta-Glycyrrhetic Acid; Enoxolone; 3β-Hydroxy-11-oxo-18β; 20β-olean-12-en-29-oic Acid; (3β,20β)-3-Hydroxy-11-oxoolean-12-en-29-oic Acid |
| Stock Status | In Stock, Commercial Production |
| CAS Number | 471-53-4 |
| Molecular Formula | C30H46O4 |
| Molecular Weight | 470.69 g/mol |
| Melting Point | 285.0~295.0℃ |
| Solubility | Practically Insoluble in Water, Soluble in Ethanol, Sparingly Soluble in Methylene Chloride |
| COA & MSDS | Available |
| Origin | Shanghai, China |
| Category | Natural Plant Extract |
| Brand | Ruifu Chemical |
| Items | Specifications | Results |
| Appearance | White or Almost White Crystalline Powder | White Crystalline Powder |
| Infrared Absorption | Complies | Complies |
| TLC | Complies | Complies |
| Solution | Clear and Not More Intensely Colored
Than Reference Solution Y6 |
Complies |
| Solubility | Soluble in Ethanol | Complies |
| Melting Point | 285.0~295.0℃ | 292.0~295.0℃ |
| Specific Rotation | +145.0° to +154.0° (Dried Substance) | +151.5° |
| Loss on Drying | <1.00% | 0.92% |
| Sulphated Ash | <0.10% | 0.05% |
| Arsenic (As) | ≤2ppm | <2ppm |
| Heavy Metals | ≤20ppm | <20ppm |
| Residual Ethanol | <0.50% | 0.23% |
| Related Substances | ||
| Any Impurity | <0.70% | Complies |
| Total Impurities | <2.00% | Complies |
| Total Plate Count | <100cfu/g | <100cfu/g |
| Yeast And Moulds | <10cfu/g | <10cfu/g |
| E. Coli | Negative | Negative |
| Samonella | Negative | Negative |
| Infrared Spectrum | Consistent with Structure | Complies |
| Assay (Potentiometry) | 98.0~101.0% (Dried Substance) | 99.3% |
| Conclusion | The product has been tested and complies with the given specifications | |
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Keep the container tightly closed and store in a cool, dry and well-ventilated warehouse away from incompatible substances. Protect from light and moisture.
Shipping: Deliver to worldwide by air, by FedEx / DHL Express. Provide fast and reliable delivery.
General Notices
(Ph Eur monograph 1511)
C30H46O4 470.7 471-53-4
Action and use
Treatment of benign peptic ulcer disease.
Ph Eur
DEFINITION
(20β)-3β-Hydroxy-11-oxo-olean-12-en-29-oic acid.
Content
98.0 per cent to 101.0 per cent (dried substance).
CHARACTERS
Appearance
White or almost white crystalline powder.
Solubility
Practically insoluble in water, soluble in ethanol, sparingly soluble in methylene chloride.
It shows polymorphism (5.9).
IDENTIFICATION
First identification A.
Second identification B, C.
A. Examine by infrared absorption spectrophotometry (2.2.24).
Comparison Enoxolone CRS.
If the spectra obtained in the solid state show differences, dissolve 0.2 g of the substance to be examined and 0.2 g of the reference substance separately in 6 ml of ethanol R. Boil under
a reflux condenser for 1 h and add 6 ml of water R. A precipitate is formed. Cool to about 10℃ and filter with the aid of vacuum. Wash the precipitate with 10 ml of alcohol R, dry in an oven at 80℃ and record new spectra.
B. Thin-layer chromatography (2.2.27).
Test solution Dissolve 10 mg of the substance to be examined in methylene chloride R and dilute to 10 ml with the same solvent.
Reference solution Dissolve 10 mg of enoxolone CRS in methylene chloride R and dilute to 10 ml with the same solvent.
Plate TLC silica gel plate R.
Mobile phase glacial acetic acid R, acetone R, methylene chloride R (5:10:90 V/V/V).
Application 5 µl.
Development Over 2/3 of the plate.
Drying In air for 5 min.
Detection Spray with anisaldehyde solution R and heat at 100-105℃ for 10 min. Results The principal spot in the chromatogram obtained with the test solution is similar in position, colour and size to the principal spot in the chromatogram obtained with the reference
solution.
C. Dissolve 50 mg in 10 ml of methylene chloride R. To 2 ml of this solution, add 1 ml of acetic anhydride R and 0.3 ml of sulphuric acid R. A pink colour is produced.
TESTS
Appearance of solution
The solution is clear (2.2.1) and not more intensely coloured than reference solution Y6 (2.2.2, Method II).
Dissolve 0.1 g in ethanol R and dilute to 10 ml with the same solvent.
Specific optical rotation (2.2.7) + 145 to + 154 (dried substance).
Dissolve 0.50 g in dioxan R and dilute to 50.0 ml with the same solvent.
Related substances
Liquid chromatography (2.2.29).
Test solution Dissolve 0.10 g of the substance to be examined in the mobile phaseand dilute to 100.0 ml with the mobile phase.
Reference solution (a) Dilute 2.0 ml of the test solution to 100.0 ml with the mobile phase.
Reference solution (b) Dilute 5.0 ml of reference solution (a) to 100.0 ml with the mobile phase.
Reference solution (c) Dissolve 0.1 g of 18α-glycyrrhetinic acid R in tetrahydrofuran R and dilute to 100.0 ml with the same solvent. To 2.0 ml of the solution, add 2.0 ml of the test solution and dilute to 100.0 ml with the mobile phase.
Column:
-size: l = 0.25 m, Ø = 4.6 mm,
-stationary phase: octadecylsilyl silica gel for chromatography R (5 µm),
-temperature: 30℃.
Mobile phase Mix 430 volumes of tetrahydrofuran R and 570 volumes of a 1.36 g/l solution of sodium acetate R adjusted to pH 4.8 with glacial acetic acid R.
Flow rate 0.8 ml/min.
Detection Spectrophotometer at 250 nm.
Injection 20 µl loop injector; inject the test solution and the reference solutions.
Run time 4 times the retention time of Enoxolone.
System suitability:
-resolution: minimum of 2.0 between the peaks due to enoxolone and to 18α-glycyrrhetinic acid in the chromatogram obtained with reference solution (c).
Limits:
-any impurity: not more than 7 times the area of the principal peak in the chromatogram obtained with reference solution (b) (0.7 per cent),
-total: not more than the area of the principal peak in the chromatogram obtained with reference solution (a) (2.0 per cent),
-disregard limit: 0.5 times the area of the principal peak in the chromatogram obtained with reference solution (b) (0.05 per cent).
Heavy metals (2.4.8) Maximum 20 ppm.
1.0 g complies with limit test F. Prepare the standard using 2 ml of lead standard solution (10 ppm Pb) R.
Loss on drying (2.2.32) Maximum 0.5 per cent, determined on 1.000 g by drying in an oven at 105℃ for 4 h.
Sulphated ash (2.4.14) Maximum 0.2 per cent, determined on 1.0 g.
ASSAY
Dissolve 0.330 g in 40 ml of dimethylformamide R. Titrate with 0.1 M tetrabutylammonium hydroxide, determining the end-point potentiometrically (2.2.20). Carry out a blank titration. 1 ml of 0.1 M tetrabutylammonium hydroxide is equivalent to 47.07 mg of C30H46O4
Crown Copyright 2006 330 46 4
STORAGE
Protected from light.
IMPURITIES
A. (20β)-3β-hydroxy-11-oxo-18α-olean-12-en-29-oic acid,
B. (4β,20β)-3β,23-dihydroxy-11-oxo-olean-12-en-29-oic acid.
Ph Eur
1, Licorice crude→Glycyrrhizic acid crude aqueous solution→Glycyrrhizic acid crude
2, Glycyrrhizic acid crude→Glycyrrhizic acid ethanol solution→Tripotassium glycyrhetate→Monopotassium glycyrrhizate
3, Monopotassium glycyrrhizate→Glycyrrhetic acid crude→Glycyrrhetic acid
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc.
Advantages? Superior quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
| Hazard Symbols | Xn - Harmful |
| Risk Codes | R22 - Harmful if swallowed |
| R36 - Irritating to the eyes | |
| Safety Description | S22 - Do not breathe dust. |
| S24/25 - Avoid contact with skin and eyes. | |
| WGK Germany | 3 |
| RTECS | RK0180000 |
| HS Code | 2938909020 |
Glycyrrhetic Acid (18β-Glycyrrhetinic Acid; Enoxolone) (CAS: 471-53-4)
Function of Glycyrrhetinic Acid
1. Has the functions of adrenocortical hormones.
2. Has the functions of resisting ulcer, inflammation and allergy.
3. Detoxify, antibiotic, obviously suppress HIV appreciation to strengthen the immunity.
4. Resist brain diseases caused by blood deficiency, preventing artery sclerosis from occurring anddevelopment.
5. It is chiefly applied to pharmaceuticals industry as the material of eyedrop and officinal toothpaste;
6. It has the efficacy in Anti-inflammatory, antianaphylaxis and moist boost.
7. It is active against conjunctivitis, keratitis, stomatitis and periodontitis.
Application of Glycyrrhetinic Acid
1. Glycyrrhetinic Acid as a sweetener,it is used in food industry.
2. Glycyrrhetinic Acid as product for benefiting stomach, it is widely used in health industry.
3. Glycyrrhetinic Acid as raw materials of drugs for clearing heat and detoxicating, it is used in medicine field.
4. Glycyrrhetinic Acid applied in cosmetic field, it able to nourish and cure the skin.
5. Glycyrrhetinic Acid is benefiting stomach, treating weakness of stomach, lassitude, shortness of breath;
6. Glycyrrhetinic Acid has the function of antibacterial, anti-inflammatory, anti-viral and promoting the immune system;
7. Glycyrrhetinic Acid can be used for clearing heat and detoxicating, expectorant and trating cough and epigastric abdominal.
-
Glycyrrhetic Acid (Enoxolone) CAS 471-53-4 Assa...
-

Tea Tree Oil CAS 68647-73-4
-
(-)-Epigallocatechin Gallate Hydrate CAS 989-51...
-
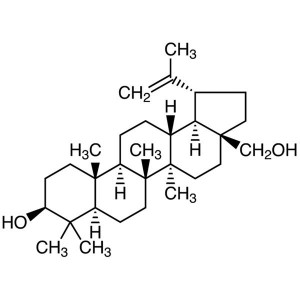
Betulin CAS 473-98-3 Purity >99.0% (HPLC) Plant...
-
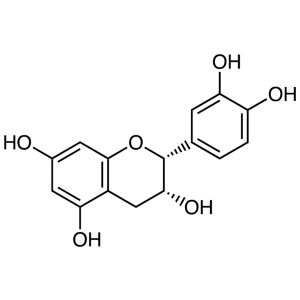
(-)-Epicatechin CAS 490-46-0 Purity ≥95.0% (HPLC)
-
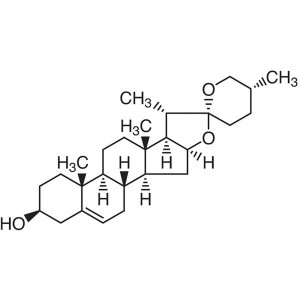
Diosgenin CAS 512-04-9 Purity >98.0% (HPLC) Wil...
-
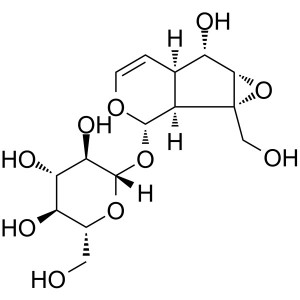
Catalpol CAS 2415-24-9 Purity ≥98.0% (HPLC) Fac...