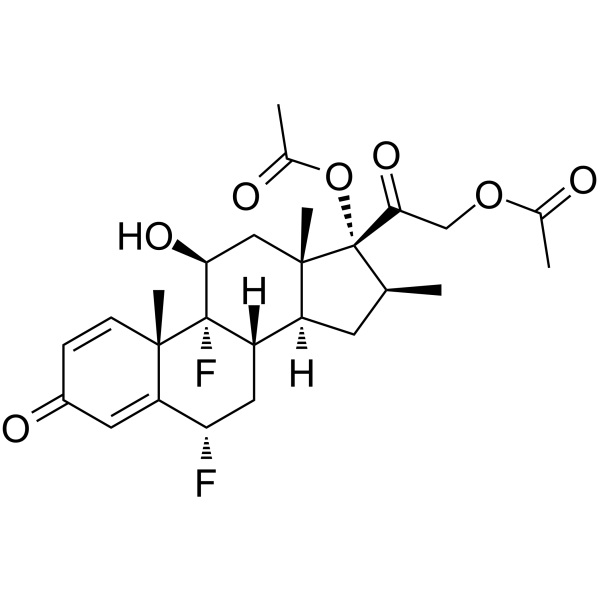
Diflorasone Diacetate CAS 33564-31-7 Assay 97.0~103.0% Factory Corticosteroid
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer of Diflorasone Diacetate (CAS: 33564-31-7) with high quality. Diflorasone Diacetate is a synthetic corticosteroid used to treat inflammatory skin diseases. Ruifu Chemical can provide worldwide delivery, competitive price, small and bulk quantities available. Purchase Diflorasone Diacetate, Please contact: alvin@ruifuchem.com
| Chemical Name | Diflorasone Diacetate |
| Synonyms | (6α,11β,16β)-17,21-Bis(acetyloxy)-6,9-difluoro-11-hydroxy-16-methyl-pregna-1,4-diene-3,20-dione; 6a,9-Difluoro-11b,17,21-trihydroxy-16b-methylpregna-1,4-diene-3,20-dione 17,21-diacetate; Dermaflor; Diacort; Diflorasone 17,21-Diacetate; Difulal; Florone; Maxiflor; Psorcon; Soriflor |
| Stock Status | In Stock, Commercial Scale |
| CAS Number | 33564-31-7 |
| Molecular Formula | C26H32F2O7 |
| Molecular Weight | 494.52 g/mol |
| Melting Point | 221.0~223.0℃(dec) |
| Solubility | Soluble in DMSO |
| Storage Temp. | Cool & Dry Place (2~8℃) |
| COA & MSDS | Available |
| Shelf Life | >3 Years if Stored Properly |
| Brand | Ruifu Chemical |
| Items | Inspection Standards | Results |
| Appearance | White to Pale Yellow Crystalline Powder | White Crystalline Powder |
| Specific Rotation [α]20/D | +58.0° to +68.0° |
+59.5° |
| Loss on Drying | <0.50% | 0.18% |
| Residue on Ignition | <0.50% | 0.03% |
| Residual Solvents | ||
| Methanol | ≤3000ppm | Conforms |
| Dichloromethane | ≤600ppm | None |
| Chloroform | ≤60ppm | None |
| Pyridine | ≤200ppm | None |
| N,N-Dimethylformamide | ≤880ppm | None |
| Chromatographic Purity | ||
| The Biggest Individual Impurity | ≤1.00% | Conforms |
| Total Impurities | ≤2.00% | 1.2% |
| Assay | 97.0~103.0% (Calculated on the Dried Basis) | 98.8% |
| Infrared Spectrum | Conforms to Structure | Conforms |
| 1 H NMR Spectrum | Conforms to Structure | Conforms |
| Conclusion | The product has been tested & complies with the USP35 | |
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Store in sealed containers at cool and dry (2~8℃) warehouse away from incompatible substances. Protect from light and moisture.
Shipping: Deliver to worldwide by FedEx / DHL Express. Provide fast and reliable delivery.
Diflorasone Diacetate [33564-31-7]
» Diflorasone Diacetate contains not less than 97.0 percent and not more than 103.0 percent of C26H32F2O7, calculated on the dried basis.
Packaging and storage-Preserve in tight containers.
USP Reference standards <11>-
USP Diflorasone Diacetate RS
Identification, Infrared Absorption <197M>.
Specific rotation <781S>: between +58° and +68°. Test solution: 20 mg, undried, per mL, in chloroform.
Loss on drying <731>-Dry it in vacuum at 60 ° and at a pressure not exceeding 5 mm of mer cury for 16 hours: it loses not more than 0.5% of its weight.
Residue on ignition <281>: not more than 0.5%
Chromatographic purity-
Mobile phase and Chromatographic system-Prepare as directed in the Assay.
Test solution-Dissolve an accurately weighed quantity of Diflorasone Diacetate in water-saturated chloroform to obtain a solution having a concentration of about 6 mg per mL.
Procedure-Inject a volume (about 10 µL) of the Test solution into the chromatograph, record the chromatogram for a period equal to 5 times the retention time of the major peak, and measure the areas of all the peaks. Calculate the percentage of each impurity in the portion of Diflorasone Diacetate taken by the formula:
100(ri / rs)
in which ri is the peak area for each impurity; and rs is the sum of the areas of all the peaks: not more than 1.0% of any individual impurity is found; and not more than 2.0% of total impurities is found.
Assay-
Mobile phase-Prepare a solution containing a mixture of water-saturated n-butyl chloride, water-saturated methylene chloride, glacial acetic acid, and tetrahydrofuran (350:125:15:10). Make adjustments if necessary (see System Suitability under Chromatography <621>).
Internal standard solution-Using water-saturated chloroform, prepare a solution of isoflupredone acetate containing about 0.04 mg per mL.
Standard preparation-Dissolve an accurately weighed quantity of USP Diflorasone Diacetate RS in Internal standard solution to obtain a solution having a known concentration of about 33µg per mL.
Assay preparation-Transfer about 15 mg of Diflorasone Diacetate, accurately weighed, to a 500-mL volumetric flask. Add Internal standard solution to volume, and mix.
Chromatographic system (see Chromatography <621>)-The liquid chromatograph is equipped with a 254-nm detector and a 4.6-mm × 10-cm column that contains 3- µm packing L3. The flow rate is about 2.5 mL per minute. Chromatograph the Standard preparation, and record the peak areas as directed for Procedure: the resolution, R, between the analyte and internal standard peaks is not less than 12; and the relative standard deviation for not less than four replicate injections is not more than 2.0%.
Procedure-Separately inject equal volumes (about 10 µL) of the Standard preparation and the Assay preparation into the chromatograph, record the chromatograms, and measure the areas for the major peaks. The relative retention times are about 1.0 for diflorasone diacetate and 2.4 for the internal standard. Calculate the quantity, in mg, of C 26H32F2O7 in the portion of Diflorasone Diacetate taken by the formula:
0.5C(RU / RS)
in which C is the concentration, in µg per mL, of USP Diflorasone Diacetate RS in the Standard preparation; and RU and RS are the ratios of the peak areas for Diflorasone Diacetate and the internal standard areas obtained from the Assay preparation and the Standard preparation, respectively.
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc.
Advantages? Superior quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
Hazard Symbols Xn - Harmful
Risk Codes
R20/21/22 - Harmful by inhalation, in contact with skin and if swallowed.
R40 - Limited evidence of a carcinogenic effect
Safety Description
S22 - Do not breathe dust.
S36 - Wear suitable protective clothing.
WGK Germany 3
RTECS TU3722000
HS Code 2937210000
Diflorasone Diacetate (CAS: 33564-31-7) is a synthetic corticosteroid used to treat inflammatory skin diseases. An anti-inflammatory and anti-itching corticosteroid usually present in topical creams. Brand name Florone (Pharmacia & Upjohn); Psorcon (Pharmacia & Upjohn); Psorcon (Sanofi Aventis). Diflorasone Diacetate Ointment. Diflorasone Diacetate reduces swelling, redness, itching, or rashes caused by skin conditions, such as eczema and psoriasis. It works by decreasing inflammation of the skin. It belongs to a group of medications called topical steroids. Diflorasone Diacetate is a prescription drug approved by the U.S. Food and Drug Administration (FDA) to treat skin inflammation and itching associated with skin conditions that respond to corticosteroids. Diflorasone diacetate has been sold under various brand names, including Apexicon, Maxiflor, Psorcon, and Florone.
-
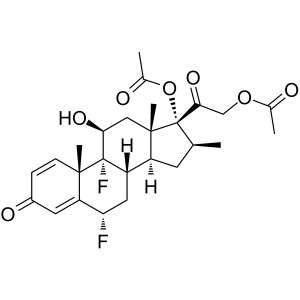
Diflorasone Diacetate CAS 33564-31-7 Assay 97.0...
-
Sorafenib Tosylate CAS 475207-59-1 Purity ≥99.0...
-
Vonoprazan Fumarate (TAK-438) CAS 1260141-27-2 ...
-
Afatinib Dimaleate CAS 850140-73-7 Purity >99.5...
-
Anastrozole CAS 120511-73-1 API Factory High Qu...
-
Aripiprazole CAS 129722-12-9 Assay 98.0%~102.0%...
-
Azelastine Hydrochloride CAS 79307-93-0 Assay 9...
-
Betamethasone CAS 378-44-9 Purity 97.0%~103.0% ...
-
Baloxavir Marboxil CAS 1985606-14-1 API Factory...
-
Bicalutamide CAS 90357-06-5 API Factory High Qu...
-
Candesartan Cilexetil CAS 145040-37-5 Purity >9...
-
Captopril CAS 62571-86-2 API Factory USP High Q...
-
Crizotinib CAS 877399-52-5 Assay ≥99.0% API Fac...
-
Daptomycin CAS 103060-53-3 Purity ≥95.0% API Fa...
-
Favipiravir CAS 259793-96-9 T-705 Purity ≥99.0%...
-
Etravirine TMC-125 CAS 269055-15-4 Assay ≥99.0%...