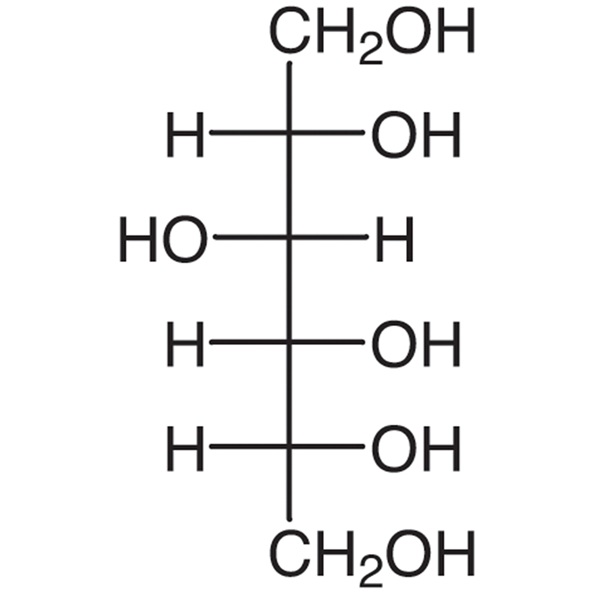
D-Sorbitol CAS 50-70-4 Assay 97.0~100.5% Factory High Quality
Shanghai Ruifu Chemical Co., Ltd. is one of the leading manufacturers and suppliers of D-Sorbitol (CAS: 50-70-4) in china with high quality, production capacity 20000 tons per year. Our D-Sorbitol is sold well both at domestic and abroad market, deeply trusted by customers. We can provide worldwide delivery, small and bulk quantities available. If you want to purchase D-Sorbitol, Please contact: alvin@ruifuchem.com
| Chemical Name | D-Sorbitol |
| Synonyms | D-(-)-Sorbitol; Sorbitol; (-)-Sorbitol; Dextro-Sorbitol; D-Glucitol; D-Sorbol; Gulitol; Esasorb; L-Gulitol |
| Stock Status | In Stock, Production Capacity 20000 Tons per Year |
| CAS Number | 50-70-4 |
| Molecular Formula | C6H14O6 |
| Molecular Weight | 182.17 |
| Melting Point | 98.0~100.0℃(lit.) |
| Density | 1.28 g/mL at 25℃ |
| Refractive Index n20/D | 1.46 |
| Sensitive | Hygroscopic |
| Water Solubility | Very Soluble in Water, Almost Transparency |
| Solubility | Insoluble in Ethanol (96 percent), Insoluble in Ether |
| Storage Temp. | Sealed in Dry, Store at Room Temperature |
| COA & MSDS | Available |
| Brand | Ruifu Chemical |
| Hazard Codes | Xi | RTECS | LZ4290000 |
| Risk Statements | 36/37/38 | F | 3 |
| Safety Statements | 8-36-26-24/25 | TSCA | Yes |
| WGK Germany | 2 | HS Code | 2905440000 |
| Items | Inspection Standards | Results |
| Appearance | White Crystalline Powder | Conforms |
| Taste | Sweet Taste, Feels Cool | Conforms |
| State of Solution | Clear and Colorless (5.0g in 20ml of H2O) | Conforms |
| Chloride (Cl) | ≤0.005% | <0.005% |
| Sulfate (SO4) | ≤0.005% | <0.005% |
| Heavy Metals (as Pb) | ≤5.0ppm | <5.0ppm |
| Nickel (Ni) | ≤1.0ppm | <1.0ppm |
| Lead (Pb) | ≤1.0ppm | <1.0ppm |
| Arsenic (As2O3) | ≤1.0ppm | <1.0ppm |
| Reducing Sugars (as Glucose) | ≤0.30% | <0.10% |
| Total Sugars | ≤0.50% | <0.50% |
| Water | ≤1.50% | <1.50% |
| Residue on Ignition (Sulfated) | ≤0.10% | <0.10% |
| D-Sorbitol Assay | 97.0~102.0% (on dried basis) | 99.2% |
| pH Value | 3.5 to 7.0 (50% aq. Solution) | 6.0 |
| Total Aerobic Count | ≤1000 cfu/g | <1000 cfu/g |
| Total Molds and Yeasts | ≤100 cfu/g | <100 cfu/g |
| Escherichia Coli | Absence | Absence |
| Salmonella | Absence | Absence |
| Bacterial Endotoxins | Conforms | Conforms |
| Infrared Spectrum | Conforms to Structure | Conforms |
| Conclusion | This Product by Inspection Accords with the Specifications | |
D-Sorbitol (CAS: 50-70-4) EP8.7 Test Method
D-Glucitol (D-sorbitol).
Content: 97.0 per cent to 102.0 per cent (anhydrous substance).
CHARACTERS
Appearance: white or almost white, crystalline powder.
Solubility: very soluble in water, practically insoluble in ethanol (96 per cent).
It shows polymorphism (5.9).
DENTIFICATION
First identification: A.
Second identification: B, C, D
A. Examine the chromatograms obtained in the assay.
Results: the principal peak in the chromatogram obtained with the test solution is similar in retention time and size to the principal peak in the chromatogram obtained with reference solution (a).
B. Dissolve 0.5 g with heating in a mixture of 0.5 mL of pyridine Rand 5 mL of acetic anhydride R. After 10 min pour the solution into 25 mL ofwater Rand allow to stand in iced water for 2 h. The precipitate, recrystallised from a small volume of ethanol (96 per cent) R and dried in vacuo, melts (2.2.14) at 98℃ to 104℃.
C. Thin-layer chromatography (2.2.27).
Test solution. Dissolve 25 mg of the substance to be examined inwater R and dilute to 10 mL with the same solvent.
Reference solution (a). Dissolve 25 mg of sorbitol CRS in water R and dilute to 10 mL with the same solvent.
Reference solution (b). Dissolve 25 mg of mannitol CRS and 25 mg of sorbitol CRS in water R and dilute to 10 mL with the same solvent.
Plate:TLC silica gel G plate R.
Mobile phase: water R, ethyl acetate R, propanol R (10:20:70V/V/V).
Application: 2 μL
Development: over a path of 17cm
Drying: in air.
Detection: spray with 4-aminobenzoic acid solution R; dry in a current of cold air until the acetone is removed; heat at 100℃ for 15min; allow to cool and spray with a 2g/L solution of sodium periodate R; dry in a current of cold air; heat at 100℃ for 15 min.
System suitability: reference solution (b):
– the chromatogram shows 2 clearly separated spots.
Results: the principal spot in the chromatogram obtained with the test solution is similar in position, colour and size to the principal spot in the chromatogram obtained with reference solution (a).
D. Specific optical rotation (2.2.7): +4.0 to +7.0 (anhydrou substance).
Dissolve 5.00 g of the substance to be examined and 6.4 g of disodium tetraborate R in 40 mL of water R. Allow to stand for 1h,shaking occasionally, and dilute to 50.0mL with water R. Filter if necessary.
TESTS
Appearance of solution.The solution is clear(2.2.1) and colourless (2.2.2, Method II).
Dissolve 5 g inwater R and dilute to 50 mL with the same solvent.
Conductivity (2.2.38): maximum 20 μS·cm−1
Dissolve 20.0 g incarbon dioxide-free water R prepared from distilled water R and dilute to 100.0 mL with the same solvent. Measure the conductivity of the solution while gently stirring with a magnetic stirrer.
Reducing sugars: maximum 0.2 per cent, expressed as glucose equivalent.
Dissolve 5.0 g in 6 mL of water Rwith the aid of gentle heat. Cool and add 20 mL of cupri-citric solution R and a few glass beads. Heat so that boiling begins after 4 min and maintain boiling for 3 min. Cool rapidly and add 100 mL of a 2.4 per cent V/V solution of glacial acetic acid R and 20.0 mL of 0.025 M iodine. With continuous shaking, add 25 mL of a mixture of 6 volumes of hydrochloric acid R and 94 volume of water R and, when the precipitate has dissolved, titrate the excess of iodine with 0.05 M sodium thiosulfate using 1 mL of starch solution R, added towards the end of the titration, as indicator. Not less than 12.8 mL of 0.05 M sodium thiosulfate is required.
Related substances. Liquid chromatography(2.2.29).
Test solution. Dissolve 5.0 g of the substance to be examined in 20 mL of water R and dilute to 100.0 mL with the same solvent.
Reference solution (a). Dissolve 0.50g of sorbitol CRS in 2 mL of water R and dilute to 10.0 mL with the same solvent.
Reference solution (b). Dilute 2.0mL of the test solution to 100.0 mL with water R.
Reference solution (c). Dilute 5.0 mL of reference solution (b) to 100.0 mL with water R.
Reference solution (d). Dissolve 0.5g of sorbitol R and 0.5 g of mannitol R (impurity A) in 5 mL of water R and dilute to 10.0 mL with the same solvent.
Column:
–size:l=0.3m,Ø=7.8mm
–stationary phase: strong cation-exchange resin (calcium form) R (9 μm);
–temperature: 85±1°C
Mobile phase: degassed water R.
Flow rate: 0.5mL/min
Detection: refractometer maintained at a constant temperature (e.g. 35 °C).
Injection: 20μL of the test solution and reference solutions (b) (c) and (d)
Run time: twice the retention time of sorbitol.
Relative retention with reference to sorbitol (retention time = about 27 min): impurity C = about 0.6; impurity A = about 0.8; impurity B = about 1.1.
System suitability: reference solution (d):
–resolution: minimum 2.0 between the peaks due to impurity A and sorbitol.
Limits:
–any impurity: for each impurity, not more than the area of the principal peak in the chromatogram obtained with reference solution (b) (2 per cent);
–total: not more than 1.5 times the area of the principal peak in the chromatogram obtained with reference solution (b) (3 per cent);
–disregard limit: the area of the principal peak in the chromatogram obtained with reference solution (c) (0.1 percent).
Lead (2.4.10): maximum 0.5 ppm.
Nickel (2.4.15): maximum 1 ppm.
Dissolve the substance to be examined in 150.0 mL of the prescribed mixture of solvents.
Water (2.5.12): maximum 1.5 per cent, determined on 1.00 g. Use a mixture of 1volume of formamide R and 2 volumes of anhydrous methanol R as solvent.
Microbial contamination
If intended for use in the manufacture of parenteral preparations:
– TAMC: acceptance criterion 102CFU/g (2.6.12).
If not intended for use in the manufacture of parenteral preparations:
– TAMC: acceptance criterion 103CFU/g (2.6.12);
– TYMC: acceptance criterion 102CFU/g (2.6.12);
–absence of Escherichia coli (2.6.13);
–absence of Salmonella (2.6.13).
Bacterial endotoxins(2.6.14). If intended for use in the manufacture of parenteral preparations without a further appropriate procedure for the removal of bacterial endotoxins:
– less than 4 IU/g for parenteral preparations having a concentration of less than 100g/L of sorbitol
– less than 2.5 IU/g for parenteral preparations having a concentration of 100g/L or more of sorbitol
ASSAY
Liquid chromatography (2.2.29) as described in the test for related substances with the following modification.
Injection: test solution and reference solution (a).
Calculate the percentage content of D-sorbitol from the declared content of sorbitol CRS.
LABELLING
The label states:
– where applicable, the maximum concentration of bacterial endotoxins;
– where applicable, that the substance is suitable for use the manufacture of parenteral preparations.
IMPURITIES
A. D-mannitol,
B. D-iditol,
C. 4-O-α-D-glucopyranosyl-D-glucitol (D-maltitol).
D-Sorbitol (CAS: 50-70-4) USP Test Method
DEFINITION
Sorbitol contains NLT 91.0% and NMT 100.5% of D-sorbitol, calculated on the anhydrous basis. The amounts of total sugars, other polyhydric alcohols, and any hexitol anhydrides, if detected, are not included in the requirements, nor in the calculated amount under Other Impurities in General Notices.
IDENTIFICATION
• A.
Sample solution: 1g of Sorbitol in 75 mL of water
Analysis: Transfer 3 mL of Sample solution to a 15-cm test tube, and add 3 mL of freshly prepared catechol solution (1 in 10), and mix. Add 6 mL of sulfuric acid, then gently heat the tube in a flame for 30 s.
• B. The retention time of the major peak of the Sample solution corresponds to that from the Standard solution, as obtained in the Assay.
ASSAY
• PROCEDURE
Mobile phase: Use degassed water.
System suitability solution: Prepare a solution containing 4.8 mg/g of each USP Sorbitol RS and mannitol
Standard solution: 4.8 mg/g of USP Sorbitol RS
Sample solution: Dissolve 0.10 g of Sorbitol in water, and dilute with water to 20g. Record the final solution weight, and mix thoroughly.
Chromatographic system
(See Chromatography <621>, System Suitability.)
Mode: LC
Detector: Refractive index
Column: 7.8-mm x 10-cm; packing L34
Temperature
Column: 50±2°
Detector: 35°
Flow rate: 0.7 mL/min
Injection size: 10 μL
System suitability
Samples: System suitability solution and Standard solution [NOTE-The relative retention times for mannitol and sorbitol are about 0.6 and 1.0, respectively.]
Suitability requirements
Resolution: NLT 2.0 between sorbitol and mannitol, System suitability solution
Relative standard deviation: NMT 2.0%, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage, on the anhydrous basis, of D-sorbitol in the portion of Sorbitol taken:
Result = (rU/rS) x (CS/CU) x (100/(100 -W)) x 100
rU= peak response from the Sample solution
rS= peak response from the Standard solution
CS= concentration of USP Sorbitol RS in the Standard solution (mg/g)
CU= concentration of Sorbitol in the Sample solution (mg/g)
W= percentage obtained in the test for Water Determination
Acceptance criteria: 91.0%–100.5% on the anhydrous basis
IMPURITIES
• LIMITOF NICKE
Sample solution: Dissolve 20.0 g of Sorbitol in diluted acetic acid, and dilute with diluted acetic acid to 150 mL.
Blank solution: 150 mL of diluted acetic acid
Standard solutions: Prepare three solutions by adding 0.5, 1.0, and 1.5 mL of nickel standard solution TS to 20.0 g of Sorbitol dissolved in diluted acetic acid, and dilute with the same solvent to 150 mL.
Instrumental conditions
(See Spectrophotometry and Light-Scattering <851>)
Mode: Atomic absorption spectrophotometry
Analytical wavelength: 232.0 nm
Lamp: Nickel hollow-cathode
Flame: Air–acetylene
Analysis
Samples: Standard solutions and Sample solution
To each sample add 2.0 mL of a saturated ammonium pyrrolidinedithiocarbamate solution (containing 10g/L of ammonium pyrrolidinedithiocarbamate) and 10.0 mL of methyl isobutyl ketone, and shake for 30 s. Protect from bright light. Allow the two layers to separate, and use the methyl isobutyl ketone layer. Set the instrument to zero using the organic layer from the Blank solution.
Concomitantly determine the absorbances of the organic layer from the Samples at least three times each. Record the average of the steady readings for each of the Standard solutions and the Sample solution. Between each measurement, aspirate the organic layer from the Blank solution, and ascertain that the reading returns to zero. Plot the absorbances of the Standard solutions and the Sample solution versus the added quantity of nickel.
Extrapolate the line joining the points on the graph until it meets the concentration axis. The distance between this point and the intersection of the axes represents the concentration of nickel in the Sample solution.
Acceptance criteria: NMT 1 ppm
• RESIDUEON IGNITION <281>: NMT 0.1%, determined on a 1.5g portion
REDUCING SUGARS
[NOTE-The amount determined in this test is not included in the calculated amount under Other Impurities in the General Notices.]
Sample solution: Dissolve 3.3 g of Sorbitol in 3 mL of water with the aid of gentle heat. Cool, and add 20.0 mL of cupric citrate TS and a few glass beads. Heat so that boiling begins after 4 min, and maintain boiling for 3 min. Cool rapidly, and add 40 mL of diluted acetic acid, 60 mL of water, and 20.0 mL of 0.05 N iodine VS. With continuous shaking, add 25 mL of a mixture of 6 mL of hydrochloric acid and 94 mL of water.
Analysis: When the precipitate has dissolved, titrate the excess of iodine with 0.05 N sodium thiosulfate VS using 2mL of starch TS, added toward the end of the titration, as an indicator.
Acceptance criteria: NLT 12.8 mL of 0.05 N sodium thiosulfate VS is required, corresponding to NMT 0.3% of reducing sugars, as glucose
• CHLORIDEAND SULFATE, Chloride<221> (if labeled for use in preparing parenteral dosage forms)
Sample: 1.5 g
Acceptance criteria: The Sample shows no more chloride than corresponds to 0.10 mL of 0.020 N hydrochloric acid (NMT 0.0050%).
• CHLORIDEAND SULFATE, Sulfate <221> (if labeled for use in preparing parenteral dosage forms)
Sample: 1.0 g
Acceptance criteria: The Sample shows no more sulfate than corresponds to 0.10 mL of 0.020 N sulfuric acid (NMT 0.01%).
SPECIFIC TESTS
• MICROBIAL ENUMERATION TESTS <61> and TESTS FOR SPECIFIED MICROORGANISMS <62>: The total aerobic count using the Plate Method is NMT 1000 cfu/g, and the total combined molds and yeasts count is NMT 100 cfu/g
• PH <791>: 3.5-7.0, in a 10% (w/w) solution in carbon dioxide-free water
• WATER DETERMINATION, Method I <921>: NMT 1.5%
CLARITYAND COLOROF SOLUTION(if labeled for use in preparing parenteral dosage forms)
Sample: 10.0 g
Analysis: Dissolve the Sample in 100.0 mL of carbon dioxide-free water.
Acceptance criteria: The solution is clear and colorless.
• BACTERIAL ENDOTOXINS TEST <85> (if labeled for use in preparing parenteral dosage forms): NMT 4 USP Endotoxin Units/g for parenteral dosage forms having a concentration of less than 100g/L of sorbitol, and NMT 2.5 USP Endotoxin Units/g for parenteral dosage forms having a concentration of 100g/L or more of sorbitol.
ADDITIONAL REQUIREMENTS
• PACKAGINGAND STORAGE: Preserve in well-closed containers. No storage requirements are specified.
• LABELING: Sorbitol intended for use in preparing parenteral dosage forms is so labeled.
USP REFERENCE STANDARDS <11>
USP Endotoxin RS
USP Sorbitol RS
Package: Fluorinated Bottle, 25kg/bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Store in sealed containers at cool, dry and ventilated warehouse away from incompatible substances. Protect from light and moisture.
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc.
Advantages? Superior quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
D-Sorbitol (CAS: 50-70-4) is a non-volatile polyhydric sugar alcohol. It is chemically stable and not easily to be oxidized by air. It is easily soluble in water, hot ethanol, methanol, isopropanol, butanol alcohol, cyclohexanol, phenol, acetone, acetic acid and dimethyl formamide. It is not easy to be fermented by various kinds of microorganism and have a excellent heat resistance. It is initially separated from the mountain strawberry by the Boussingault (French) et al. The pH value of the saturated aqueous solution is 6 to 7. It is isomer of mannitol, Taylor alcohol, and galactose alcohol. It has a refreshing sweet taste with sweetness being 65% of sucrose. It has excellent moisture absorption capability with a low calorific value and has very wide range of effects on the food, cosmetic, pharmaceutical field. When applied in food, it can prevent the food drying, aging, and can extend the shelf life of products as well as effectively prevent the precipitation of sugars and salts contained in the foods and thus maintain the strength balance of sweetness, sour, bitter and enhance food flavor. It can be synthesize from the hydrogenation of glucose under heating and high pressure with the existence of nickel catalyst.
Main Usages of D-Sorbitol Powder
1. Daily Chemical Industry
Sorbitol can be used as an excipient, moisturizing agents, and antifreeze agents in toothpaste, with the added amount being up to 25 to 30%. This can help maintain the lubrication, color, and good taste for the paste. In cosmetics field, it is used as an anti-drying agent (substitute glycerol) which can enhance the stretch and lubricity of emulsifier, and thus is suitable for long-term storage; Sorbitan esters and sorbitan fatty acid ester as well as its ethylene oxide adducts having a advantage of a small skin irritation which is thus widely used in the cosmetics industry.
2. The Food Industry
Sorbitol is a hygroscopic, water-retaining agent that is used in the production of chewing gum and confectionery to keep food soft, improve tissue and reduce hardening of sand. Widely applies to food, uses in to manufacture the nonsugar candy and the health care class sheeting (contains piece). Adding sorbitol into foods can prevent the drying of food and make food stay fresh and soft. Application in bread cake has a significant effect. Sweetener or anti-sticking agent of sugar-free chewing gum, chocolate flavored icing for making ice cream and candy, used in beverages, candy, baking and other foods
The sweetness of sorbitol is lower than that of sucrose, and can’t be exploited by any bacteria. It is an important raw material for production of sugar-free candy and a variety of anti-caries food. Since the metabolism of the product does not cause increase of blood sugar, it can also be applied as a sweetener agent and nutrient agent for the food of patients with diabetes.
Sorbitol does not contain an aldehyde group and is not easily oxidized. It will not have Maillard reaction with amino acids upon heating. It also has certain physiological activity. It can prevent the denaturation of the carotenoids and edible fats and protein; adding this product to the concentrated milk can extend the shelf life; it can also be used to improve the color, flavor and taste of small intestine and has significant stabilizing effect and long-term storage effect on fish pate. Similar effect can also be observed in the jam.
3. The Pharmaceutical Industry
Sorbitol can be used as raw material in vitamin C; also can be used as feed syrup, injection fluids, and raw material of medicine tablet; as a drug dispersion agent and fillers, cryoprotectants, anti-crystallizing agent, medicine stabilizers, wetting agents, capsules plasticized agents, sweetening agents, and ointment matrix.
Sorbitol is also used in the manufacture of softgel capsules to store single doses of liquid medicines.A treatment for hyperkalaemia (elevated blood potassium) uses sorbitol and the ion-exchange resin sodium polystyrene sulfonate.
4. The Chemical Industry
Sorbitol abietin is often used as the raw material for common architectural coatings, also used as plasticizers and lubricants for application in polyvinyl chloride resin and other polymers.
It can from complex with iron, copper, and aluminum ion in alkaline solution to be applied to the washing and bleaching in textile industry.
Using sorbitol and propylene oxide as a starting material can produce rigid polyurethane foam as well as have some flame retardant properties.
5. The Cosmetics Industry:
In cosmetics profession widespread application.
The sorbitol is one kind of versatility industrial chemicals, it has the extremely widespread function in food, daily chemical, medicine etc, and can be used as may take the sweet taste,excipient,antiseptic etc, simultaneously has the polyols nutrition superiority, such as low heat value, low sugar, guard against effect and so on.
6. D-Sorbitol can be used as a sweetener for diabetes patients. It has good heat preservation, acid resistance and non-fermentability.
7. Others:
To replace glycerin to produce toothpaste, mannitol polyurethane, mannitol anhydride oleate, electrolytic solution of electrolytic capacitors, and a good culture medium for certain microorganisms.
-
D-Sorbitol CAS 50-70-4 Assay 97.0~100.5% Factor...
-
D-(-)-Lactic Acid CAS 10326-41-7 Assay 89.0%~91...
-
D-(-)-Leucinol CAS 53448-09-2 Purity >98.5% (GC...
-
D-(-)-Ribose CAS 50-69-1 Assay 97.0~102.0% Fact...
-
D-(-)-Tartaric Acid CAS 147-71-7 Assay 99.5%~10...
-
Diethyl D-(-)-Tartrate CAS 13811-71-7 Purity ≥9...
-
Dimethyl D-(-)-Tartrate CAS 13171-64-7 Optical ...
-
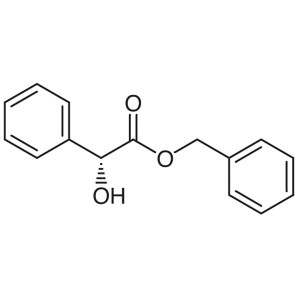
Benzyl D-(-)-Mandelate CAS 97415-09-3 Assay ≥98...
-
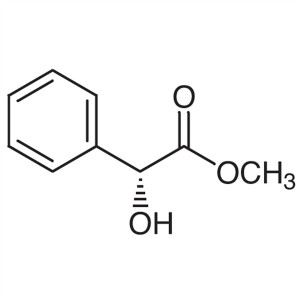
(R)-(-)-Methyl Mandelate ; Methyl D-(-)-Mandela...
-
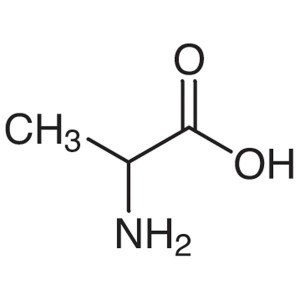
DL-Alanine CAS 302-72-7 (H-DL-Ala-OH) Assay 98....
-
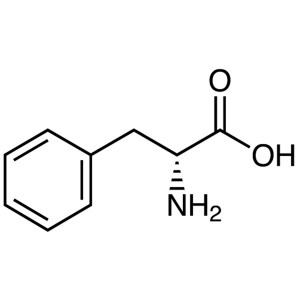
D-Phenylalanine CAS 673-06-3 (H-D-Phe-OH) Assay...
-
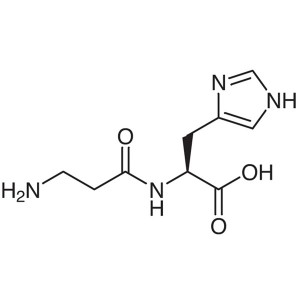
L-Carnosine CAS 305-84-0 (β-Alanyl-L-Histidine)...
-
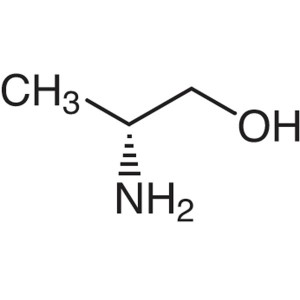
D-Alaninol CAS 35320-23-1 (H-D-Ala-Ol) Purity >...
-
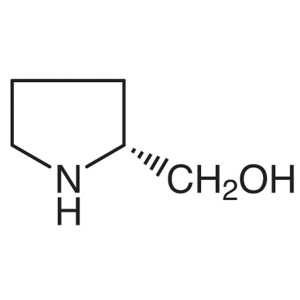
D-Prolinol CAS 68832-13-3 Purity >99.0% (GC) E....
-
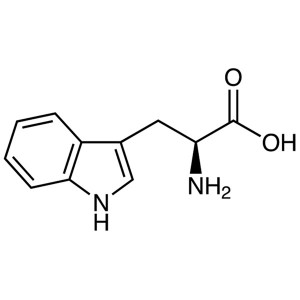
L-Tryptophan CAS 73-22-3 (H-Trp-OH) Assay 98.5~...
-
Inosine CAS 58-63-9 Assay 98.0~102.0% Factory H...