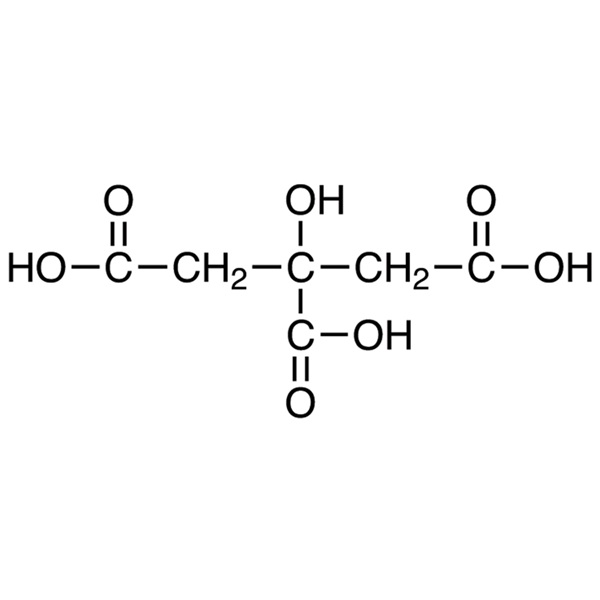
Citric Acid Anhydrous CAS 77-92-9 Assay 99.5~100.5%
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer of Citric Acid Anhydrous (CAS: 77-92-9) with high quality. Ruifu Chemical can provide worldwide delivery, competitive price, excellent service, small and bulk quantities available. Purchase Citric Acid Anhydrous, Please contact: alvin@ruifuchem.com
| Chemical Name | Citric Acid Anhydrous |
| Synonyms | 2-Hydroxy-1,2,3-Propanetricarboxylic Acid; 2-Hydroxypropan-1,2,3-Tricarboxylic Acid; 2-Hydroxypropane-1,2,3-Tricarboxylate |
| Stock Status | Bulk Stock, Sales Promotion |
| CAS Number | 77-92-9 |
| Molecular Formula | C6H8O7 |
| Molecular Weight | 192.12 g/mol |
| Melting Point | 153.0~159.0℃(lit.) |
| Density | 1.67 g/cm3 at 20℃ |
| Refractive Index n20/D | 1.493~1.509 |
| Sensitive | Hygroscopic |
| Water Solubility | Soluble in Water, 590 g/l 20℃ |
| Solubility in Methanol | Almost Transparency |
| Solubility | Very Soluble in Ethanol; Soluble in Ether; Insoluble in Chloroform, Benzene |
| Odor | Odorless |
| Stability | Stable. Incompatible with Bases, Strong Oxidizing Agents, Reducing Agents, Metal Nitrates |
| COA & MSDS | Available |
| Sample | Available |
| Origin | Shanghai, China |
| Product Categories | Food Additives |
| Brand | Ruifu Chemical |
| Items | Inspection Standards | Results |
| Appearance | White Crystalline Powder or Colorless Crystals | Complies |
| Light Transmittance | ≥96.0% | 99.0% |
| Water by Karl Fischer | ≤0.50% | 0.2% |
| Insoluble Matter in H2O | ≤0.005% | <0.005% |
| Ignition Residue (as Sulfate) | ≤0.02% | <0.02% |
| Readily Carbonizable Substances | ≤1.00% | 0.42% |
| Sulphated Ash | ≤0.05% | <0.05% |
| Chloride (Cl-) | ≤0.005% | <0.005% |
| Sulfate (SO42-) | ≤0.01% | <0.002% |
| Oxalate (as Oxalic Acid) | ≤0.01% | <0.01% |
| Calcium Salt | ≤0.02% | <0.02% |
| Iron (Fe) | ≤5mg/kg | <5mg/kg |
| Arsenic Salt | ≤1mg/kg | <1mg/kg |
| Lead (Pb) | ≤0.5mg/kg | <0.5mg/kg |
| Phospate (PO4) | ≤0.001% | <0.001% |
| Assay | 99.5%~100.5% | 99.81% |
| Infrared Spectrum | Conforms to Structure | Complies |
| Conclusion | The product has been tested & complies with the standard of GB1987-2007 | |
| Shelf Life | 2 Years From Manufacture Date if Stored Properly | |
Package: Bottle, Aluminium foil bag, 25 kg multiply paper bags with PE-Inliner, 25kg/cardboard drum, or according to customer's requirement.
Storage Condition: Keep the container tightly closed and store in a cool, dry and well-ventilated warehouse away from incompatible substances. Away from direct sunlight and water.
Shipping: Deliver to worldwide by air, by FedEx / DHL Express. Provide fast and reliable delivery.
Citric acid [77-92-9].
DEFINITION
Anhydrous Citric Acid contains NLT 99.5% and NMT 100.5% of C6H8O7, calculated on the anhydrous basis.
IDENTIFICATION
• Infrared Absorption <197K>: Dry the substance to be examined at 105℃ for 2 h.
ASSAY
• Procedure
Sample: 0.550 g of Anhydrous Citric Acid; record weight accurately.
Analysis: Dissolve the Sample in 50 mL of water. Add 0.5 mL of phenolphthalein TS. Titrate with 1 N sodium hydroxide VS. Each mL of 1 N sodium hydroxide is equivalent to 64.03 mg of C6H8O7.
Acceptance criteria: 99.5%-100.5% on the anhydrous basis
IMPURITIES
Inorganic Impurities
• Residue on Ignition <281>: NMT 0.1%, determined on 1.0 g
• Heavy Metals <231>: NMT 10 ppm
• Sulfate
Standard sulfate solution A: 1.81 mg/mL of potassium sulfate in 30% alcohol. Immediately before use, transfer 10.0 mL of this solution to a 1000-mL volumetric flask, dilute with 30% alcohol to volume, and mix. This solution contains 10 µg/mL of sulfate.
Standard sulfate solution B: 1.81 mg/mL of potassium sulfate in water. Immediately before use, transfer 10.0 mL of this solution to a 1000-mL volumetric flask, dilute with water to volume, and mix. This solution contains 10 µg/mL of sulfate.
Sample stock solution: 66.7 mg/mL of citric acid
Sample solution: To 4.5 mL of Standard sulfate solution A, add 3 mL of a barium chloride solution (1 in 4), shake, and allow to stand for 1 min. To 2.5 mL of the resulting suspension, add 15 mL of the Sample stock solution and 0.5 mL of 5 N acetic acid, and mix.
Standard solution: Prepare as directed for the Sample solution, except use 15 mL of Standard sulfate solution B instead of the Sample stock solution.
Analysis
Samples: Standard solution and Sample solution
Acceptance criteria: Any turbidity produced in the Sample solution after 5 min standing is not greater than that produced in the Standard solution (0.015%).
• Limit of Aluminum (where it is labeled as intended for use in dialysis)
Standard aluminum solution: To 352 mg of aluminum potassium sulfate in a 100-mL volumetric flask, add a few mL of water, swirl to dissolve, add 10 mL of diluted sulfuric acid, dilute with water to volume, and mix. Immediately before use, dilute 1.0 mL of this solution with water to 100.0 mL.
pH 6.0 acetate buffer: Dissolve 50 g of ammonium acetate in 150 mL of water, adjust with glacial acetic acid to a pH of 6.0, dilute with water to 250 mL, and mix.
Standard solution: Prepare a mixture of 2.0 mL of Standard aluminum solution, 10 mL of pH 6.0 acetate buffer, and 98 mL of water. Extract this mixture as described for the Sample solution, dilute the combined extracts with chloroform to volume, and mix.
Sample solution: Dissolve 20.0 g of Anhydrous Citric Acid in 100 mL of water, and add 10 mL of pH 6.0 acetate buffer. Extract this solution with successive portions of 20, 20, and 10 mL of a 0.5% solution of 8-hydroxyquinoline in chloroform, combining the chloroform extracts in a 50-mL volumetric flask. Dilute the combined extracts with chloroform to volume, and mix.
Blank solution: Prepare a mixture of 10 mL of pH 6.0 acetate buffer and 100 mL of water. Extract this mixture as described for Sample solution, dilute the combined extracts with chloroform to volume, and mix.
Fluorometric conditions
Excitation wavelength: 392 nm
Emission wavelength: 518 nm
Analysis
Samples: Standard solution and Sample solution
Determine the fluorescence intensities of the Samples in a fluorometer set as directed under Fluorometric conditions, using the Blank solution to set the instrument to zero.
Acceptance criteria: The fluorescence of the Sample solution does not exceed that of the Standard solution (0.2 ppm).
Organic Impurities
• Procedure: Limit of Oxalic Acid
Sample stock solution: 200 mg/mL of Anhydrous Citric Acid in water
Sample solution: To 4 mL of Sample stock solution add 3 mL of hydrochloric acid and 1 g of granular zinc, boil for 1 min, and allow to stand for 2 min. Transfer the supernatant to a test tube containing 0.25 mL of a phenylhydrazine hydrochloride solution (1 in 100), and heat to boiling. Cool rapidly, transfer to a graduated cylinder, and add an equal volume of hydrochloric acid and 0.25 mL of a potassium ferricyanide solution (1 in 20). Shake, and allow to stand for 30 min.
Standard solution: Prepare as directed for the Sample solution, except use 4 mL of 0.10 mg/mL oxalic acid solution, equivalent to 0.0714 mg/mL of anhydrous oxalic acid, instead of the Sample stock solution. [Note—Prepare concomitantly with the Sample solution. ]
Analysis
Samples: Standard solution and Sample solution
Acceptance criteria: Any pink color produced in the Sample solution is not more intense than that produced in the Standard solution (0.036%).
SPECIFIC TESTS
• Bacterial Endotoxins Test 85: The level of bacterial endotoxins is such that the requirement in the relevant dosage form monograph(s) in which Anhydrous Citric Acid is used can be met. Where the label states that Anhydrous Citric Acid must be subjected to further processing during the preparation of injectable dosage forms, the level of bacterial endotoxins is such that the requirement in the relevant dosage form monograph(s) in which Anhydrous Citric Acid is used can be met.
• Clarity of Solution
[Note-The Sample solution is to be compared to Standard suspension A in diffused daylight 5 min after preparation of Standard suspension A. ]
Hydrazine sulfate solution: 10 mg/mL of hydrazine sulfate in water. Allow to stand for 4 to 6 h before use.
Methenamine solution: Transfer 2.5 g of methenamine to a 100-mL glass-stoppered flask, add 25.0 mL of water, insert the glass stopper, and mix to dissolve.
Primary opalescent suspension: Transfer 25.0 mL of Hydrazine sulfate solution to the 25.0 mL of Methenamine solution in the 100-mL glass-stoppered flask. Mix, and allow to stand for 24 h. [Note—This suspension is stable for 2 months, provided it is stored in a glass container free from surface defects. The suspension must not adhere to the glass and must be well mixed before use. ]
Opalescence standard: Dilute 15.0 mL of Primary opalescent suspension with water to 1000 mL. [Note—This suspension should not be used beyond 24 h after preparation. ]
Standard suspension A: Dilute 5.0 mL of Opalescence standard with water to 100 mL.
Standard suspension B: Dilute 10.0 mL of Opalescence standard with water to 100 mL.
Sample solution: 200 mg/mL of Anhydrous Citric Acid in water
Analysis
Samples: Standard suspension A, Standard suspension B, water, and Sample solution
Transfer a sufficient portion of the Sample solution to a test tube of colorless, transparent, neutral glass with a flat base and an internal diameter of 15–25 mm to obtain a depth of 40 mm. Similarly transfer portions of Standard suspension A, Standard suspension B , and water to separate matching test tubes. Compare the Sample solution, Standard suspension A, Standard suspension B, and water in diffused daylight, viewing vertically against a black background (see Spectrophotometry and Light-Scattering 851, Visual Comparison). [Note—The diffusion of light must be such that Standard suspension A can readily be distinguished from water, and that Standard suspension B can readily be distinguished from Standard suspension A. ]
Acceptance criteria: The Sample solution shows the same clarity as that of water.
• Color of Solution
Standard stock solution A: Ferric chloride CS, cobaltous chloride CS, and dilute hydrochloric acid (10 g/L) (2.4:0.6:7.0)
Standard stock solution B: Ferric chloride CS, cobaltous chloride CS, cupric sulfate CS, and dilute hydrochloric acid (10 g/L) (2.4:1.0:0.4:6.2)
Standard stock solution C: Ferric chloride CS, cobaltous chloride CS, and cupric sulfate CS (9.6:0.2:0.2)
[Note-Prepare the Standard solutions immediately before use. ]
Standard solution A: Dilute 2.5 mL of Standard stock solution A with dilute hydrochloric acid (10 g/L) to 100 mL.
Standard solution B: Dilute 2.5 mL of Standard stock solution B with dilute hydrochloric acid to (10 g/L) 100 mL.
Standard solution C: Dilute 0.75 mL of Standard stock solution C with dilute hydrochloric acid (10 g/L) to 100 mL.
Sample solution: Use the Sample solution prepared as directed in the test for Clarity of Solution.
Analysis
Samples: Standard solution A, Standard solution B, Standard solution C, water, and Sample solution
Transfer a sufficient portion of the Sample solution to a test tube of colorless, transparent, neutral glass with a flat base and an internal diameter of 15–25 mm to obtain a depth of 40 mm. Similarly transfer portions of Standard solution A, Standard solution B, Standard solution C, and water to separate matching test tubes. Compare the Sample solution, Standard solution A, Standard solution B, Standard solution C, and water in diffused daylight, viewing vertically against a white background (see Spectrophotometry and Light-Scattering 851, Visual Comparison).
Acceptance criteria: The Sample solution is not more intensely colored than Standard solution A, B, or C, or water.
• Readily Carbonizable Substances
Sample: 1.0 g of powdered Anhydrous Citric Acid
Analysis: Transfer the Sample to a 22- × 175-mm test tube previously rinsed with 10 mL of sulfuric acid and allowed to drain for 10 min. Add 10 mL of sulfuric acid, agitate until solution is complete, and immerse in a water bath at 90 ± 1 for 60 ± 0.5 min, keeping the level of the acid below the level of the water during the entire period. Cool the tube in running water, and transfer the acid to a color-comparison tube.
Acceptance criteria: The color of the acid is not darker than that of a similar volume of Matching Fluid K (see Color and Achromicity 631) in a matching tube, the tubes being observed vertically against a white background.
• Sterility Tests 71: Where the label states that Anhydrous Citric Acid is sterile, it meets the requirements for Sterility Tests 71 in the relevant dosage form monograph(s) in which Anhydrous Citric Acid is used.
• Water Determination, Method I 921: NMT 1.0%
ADDITIONAL REQUIREMENTS
• Packaging and Storage: Preserve in tight containers. No storage requirements specified.
• Labeling: Where it is intended for use in dialysis solutions, it is so labeled. Where Anhydrous Citric Acid must be subjected to further processing during the preparation of injectable dosage forms to ensure acceptable levels of bacterial endotoxins, it is so labeled. Where Anhydrous Citric Acid is sterile, it is so labeled.
• USP Reference Standards 11
USP Citric Acid RS Click to View Structure
USP Endotoxin RS
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc.
Advantages? Superior quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
Risk Codes
R41 - Risk of serious damage to eyes
R36/37/38 - Irritating to eyes, respiratory system and skin.
R36/38 - Irritating to eyes and skin.
R37/38 - Irritating to respiratory system and skin.
R34 - Causes burns
R36 - Irritating to the eyes
R35 - Causes severe burns
R61 - May cause harm to the unborn child
R60 - May impair fertility
Safety Description
S26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S39 - Wear eye / face protection.
S37/39 - Wear suitable gloves and eye/face protection
S24/25 - Avoid contact with skin and eyes.
S36/37/39 - Wear suitable protective clothing, gloves and eye/face protection.
S45 - In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
S36 - Wear suitable protective clothing.
S53 - Avoid exposure - obtain special instructions before use.
UN IDs UN 1789 8/PG 3
WGK Germany 1
RTECS GE7350000
FLUKA BRAND F CODES 9
TSCA Yes
HS Code 2918140000
Toxicity LD50 in mice, rats (mmol/kg): 5.0, 4.6 i.p. (Gruber, Halbeisen)
Citric Acid is a weak organic acid with the formula C6H8O7. It is a natural preservative / conservative and is also used to add an acidic, or sour, taste to foods and soft drinks. In biochemistry, the conjugate base of citric acid, citrate, is important as an intermediate in the citric acid cycle, which occurs in the metabolism of all aerobic organisms. Citric acid is a commodity chemical, it is used mainly as an acidifier, as a flavoring, and as a chelating agent.
Citric Acid is a good buffering agent for solutions between about pH 2 and pH 8. It is popular in many buffers in many techniques, electrophoresis (SSC Buffer #), to stop reactions, for biopurifications, crystallography... In biological systems around pH 7, the two species present are the citrate ion and mono-hydrogen citrate ion. the pH of a 1 mM solution of citric acid will be about 3.2.
Citric Acid is an important organic acid, often containing a molecule of crystal water, odorless, with a strong sour taste, easily soluble in water. It has many uses in industry, food industry, cosmetic industry, etc.
Citric Acid is mainly used as a acidulant in food, and also used in the preparation of pharmaceutical cooling agents, detergent additives used as experimental reagents, Chromatographic Reagents and biochemical reagents, also used in the preparation of buffer. It is used in the food industry in particular as an acidifying agent, a PH buffering agent, and together with other compounds as a preservation agent. In the detergent industry, it is an ideal substitute for phosphate. Boiler chemical cleaning pickling agent, boiler chemical cleaning rinsing agent. Mainly used for food acid, also used for the preparation of medicine cooling agent, detergent with citric acid is the most functional, the most widely used acid agent. High solubility, strong chelating ability to metal ions, can be used for all kinds of food, according to the production needs of appropriate use. In addition, this product can also be used as antioxidant Synergist, compound potato starch bleach synergist and preservative. It is widely used as an acidulant and a pharmaceutical additive for foods and beverages. Can also be used as cosmetics, metal cleaning agents, mordant, non-toxic plasticizer and Boiler Scale Inhibitor of raw materials and additives. Its main salt products are sodium citrate, calcium and ammonium salts, etc., sodium citrate is blood anticoagulant, ferric ammonium citrate can be used as blood medicine. used as analytical reagent with citric acid.
Used as experimental reagents, chromatographic analysis reagents and biochemical reagents, also used in the preparation of buffer. It is used in the food industry in particular as an acidifying agent, a PH buffering agent, and together with other compounds as a preservation agent. In the detergent industry, it is an ideal substitute for phosphate. Boiler chemical cleaning pickling agent, boiler chemical cleaning rinsing agent. It is mainly used as a sour agent for food, and is also used for the preparation of medical coolants and detergents.
In terms of food additives, it is mainly used in refreshing drinks and pickled products such as carbonated drinks, fruit juice drinks, and lactic acid drinks. Its demand is subject to changes in seasonal climate. Citric acid accounts for about 2/3 of the total consumption of sour agents.
1. Adding citric acid to canned fruit can maintain or improve the flavor of the fruit, increase the acidity (reduce the pH value) of some fruits with low acidity when stored in cans, weaken the heat resistance of microorganisms and inhibit their growth, and prevent low acidity. Bacterial expansion and damage often occur in canned fruit.
2. Adding citric acid to the candy as a sour agent is easy to harmonize with the fruity taste. The use of citric acid in gel food such as jam and jelly can effectively reduce the negative charge of pectin, so that the hydrogen bonds between pectin molecules can be gelled.
3. When processing canned vegetables, some vegetables are alkaline, and using citric acid as a pH regulator can not only play a seasoning role, but also maintain its quality.
4. Citric acid has the characteristics of chelation and pH adjustment, so that it can increase the performance of antioxidant, inhibit enzyme activity and prolong the shelf life of food in the processing of quick-frozen food.
Effervescence is a popular drug delivery system for oral ingredients, citric acid reacts with sodium carbonate or aqueous sodium bicarbonate to produce large amounts of CO2 (I. E., effervescence) and sodium citrate, which allows the active ingredients in the drug to rapidly dissolve and enhance taste. For example, laxatives and analgesics have a solubilizing effect. Citric acid syrup is Fever patients with a cool drink, with flavor, cooling, detoxification effect. citric acid is widely used in various nutritional oral liquids, etc., buffering pH 3.5~4.5, maintaining the stability of active ingredients, strengthening the effect of preservatives. The combination of citric acid and fruit flavor gives people a favorite flavor to mask the bitter taste of drugs, especially traditional Chinese medicine. Adding 0.02% citric acid in liquid ingredients can form a complex of trace iron and copper, delay the degradation of active ingredients. The use of 0.1%~0.2% citric acid in the chewing tablet can improve the flavor of the tablet and make it have a lemon flavor.
Citric Acid has astringent and anti-oxidant properties. It can also be used as a product stabilizer, pH adjuster, and preservative with a low sensitizing potential. It is not usually irritating to normal skin, but it can cause burning and redness when applied to chapped, cracked, or otherwise inflamed skin. It is derived from citrus fruits.
Citric Acid reacts with oxidizing agents, bases, reducing agents and metal nitrates . Reactions with metal nitrates are potentially explosive. Heating to the point of decomposition causes emission of acrid smoke and fumes [Lewis].
Poison by intravenous route. Moderately toxic by subcutaneous and intraperitoneal routes. Mildly toxic byingestion. A severe eye and moderate skin irritant. An irritating organic acid, some allergenic properties. Combustible liquid. Potentially explosive reaction with metal nitrates. When heated to decomposition it emits acrid smoke and fumes.