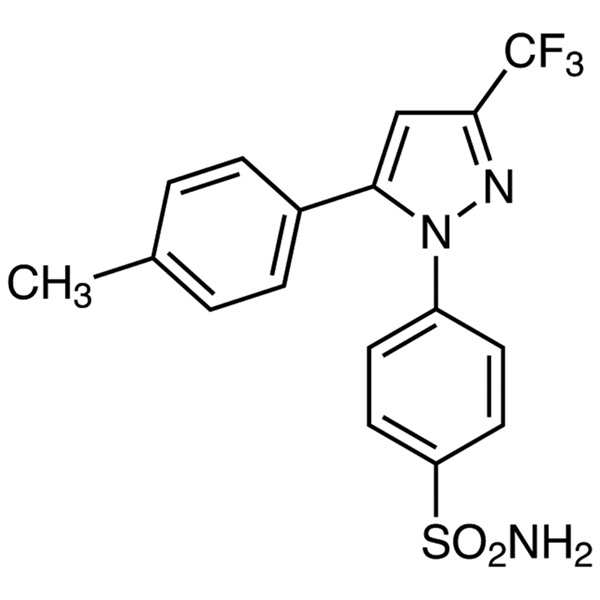
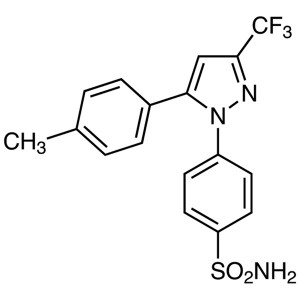
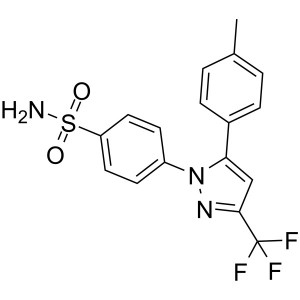
Celecoxib CAS 169590-42-5 Assay 98.0~102.0%
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer of Celecoxib (CAS: 169590-42-5) with high quality. Ruifu Chemical can provide worldwide delivery, competitive price, excellent service, small and bulk quantities available. Purchase Celecoxib, Please contact: alvin@ruifuchem.com
| Chemical Name | Celecoxib |
| Synonyms | Celebrex; Celebra; Celecox; Onsenal; Solexa; SC-58635; SC 58635; YM-177; 4-[5-(4-Methylphenyl)-3-(Trifluoromethyl)-1H-Pyrazol-1-yl]benzenesulfonamide; 5-(4-Methylphenyl)-1-(4-Sulfamoylphenyl)-3-(Trifluoromethyl)pyrazole |
| Stock Status | In Stock, Commercial Production |
| CAS Number | 169590-42-5 |
| Molecular Formula | C17H14F3N3O2S |
| Molecular Weight | 381.37 g/mol |
| Melting Point | 160.0 to 165.0℃ |
| Density | 1.43±0.10 g/cm3 |
| Water Solubility | Insoluble in Water |
| Solubility | Very Soluble in Methanol; Soluble in Ethanol |
| COA & MSDS | Available |
| Sample | Available |
| Origin | Shanghai, China |
| Brand | Ruifu Chemical |
| Items | Inspection Standards | Results |
| Appearance | White or Almost White Crystalline Powder | Complies |
| Assay | 98.0~102.0% | 99.8% |
| Melting Point | 160.0 to 165.0℃ | 162.2℃ |
| Water by Karl Fischer | <0.50% | 0.11% |
| Residue on Ignition | <0.20% | 0.05% |
| Heavy Metals (Pb) | ≤20ppm | <10ppm |
| Celecoxib Related Compound A | <0.40% | <0.20% |
| Celecoxib Related Compound B | <0.10% | <0.10% |
| Individual Unspecified Impurity | <0.10% | <0.10% |
| Total impurities | <0.50% | 0.20% |
| Infrared Spectrum | Consistent with Structure | Complies |
| 1H NMR Spectrum | Consistent with Structure | Complies |
| Conclusion | The product has been tested and complies with the given specifications | |
Package: Fluorinated Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Keep the container tightly closed and store in a cool, dry (2~8℃) and well-ventilated warehouse away from incompatible substances. Protect from light and moisture.
Shipping: Deliver to worldwide by air, by FedEx / DHL Express. Provide fast and reliable delivery.
Celecoxib
C17H14F3N3O2S 381.4
4-[5-(4-Methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]benzenesulfonamide;
p-[5-p-Tolyl-3-(trifluoromethyl)pyrazol-1-yl]benzenesulfonamide [169590-42-5]
DEFINITION
Celecoxib contains NLT 98.0% and NMT 102.0% of C17H14F3N3O2S, calculated on the anhydrous basis
IDENTIFICATION
• A. INFRARED ABSORPTION <197>: [NOTE-Methods <197A>, <197K>, or <197M> under Infrared Absorption may be used.]
[NOTE-If the spectra obtained show differences, dissolve the substance to be examined and the Reference Standard separately in isopropyl alcohol, evaporate to dryness, and record the new spectra.]
• B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
ASSAY
• PROCEDURE
Buffer: 2.7 g/L of monobasic potassium phosphate adjusted with phosphoric acid to a pH of 3.0 ± 0.2
Mobile phase: Methanol, acetonitrile, and Buffer (3:1:6)
Diluent: Methanol and water (3:1)
System suitability solution: 0.5 mg/mL of USP
Celecoxib RS and 2.4 µg/mL each of USP Celecoxib Related Compound A RS and USP Celecoxib Related Compound B RS in Diluent
Standard solution: 0.5 mg/mL of USP Celecoxib RS in Diluent
Sample solution: 0.5 mg/mL of Celecoxib in Diluent
Chromatographic system
(See Chromatography <621>, System Suitability.)
Mode: LC
Detector: UV 215 nm
Column: 4.6-mm × 25-cm; 5-µm packing L11
Column temperature: 60°
Flow rate: 1.5 mL/min
Injection size: 25 µL
Run time: About 1.5 times the celecoxib peak elution
System suitability
Samples: System suitability solution and Standard solution
Suitability requirements
Resolution: NLT 1.8 between celecoxib related compound A and Celecoxib and NLT 1.8 between Celecoxib and Celecoxib related compound B, System suitability solution
Relative standard deviation: NMT 0.73%, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of C17H14F3N3O2S in the portion of Celecoxib taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response from the Sample solution
rS = peak response from the Standard solution
CS = concentration of the Standard solution(mg/mL)
CU = concentration of the Sample solution (mg/mL)
Acceptance criteria: 98.0%~102.0% on the anhydrous basis
IMPURITIES
Inorganic Impurities
• HEAVY METALS: NMT 20 ppm
Diluent: Acetone and water (17:3)
Standard solution: Dilute 1.0 mL of Standard Lead Solution, prepared as directed under Heavy Metals <231>, Special Reagents, with Diluent to 20 mL.
Sample solution: Dissolve 0.50 g of Celecoxib in 20mL of Diluent.
Blank solution: 20 mL of Diluent
Analysis
Samples: Standard solution, Blank solution, and Sample solution
To each solution, add 2 mL of pH 3.5 Acetate Buffer prepared as directed under Heavy Metals <231>, Method I. Mix, and add to each solution 1.2 mL of thioacetamide–glycerin base TS. Mix immediately, and allow to stand for 2 min. Pass the solutions through a filter of 0.45-µm pore size. Compare the spots on the filters obtained from each of the solutions.
Acceptance criteria: The brownish-black color of the spot resulting from the Sample solution is not more intense than that of the spot resulting from the Standard solution. The test is invalid if the Standard solution does not show a brownish-black color compared to the Blank solution.
• RESIDUE ON IGNITION <281>: NMT 0.2%, using a platinum crucible
Organic Impurities
• PROCEDUR
Buffer, Mobile phase, Diluent, System suitability solution, Sample solution, and Chromatographic system: Proceed as directed in the Assay.
Standard solution: 0.5 µg/mL of USP Celecoxib RS in Diluent
System suitability
Samples: System suitability solution and Standard solution
Suitability requirements
Resolution: NLT 1.8 between Celecoxib related compound A and Celecoxib and NLT 1.8 between Celecoxib and Celecoxib related compound B, System suitability solution
Signal-to-noise ratio: NLT 20, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of each impurity in the portion of Celecoxib taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response for each impurity in the Sample solution
rS = peak response of celecoxib in the Standard solution
CS = concentration of celecoxib in the Standard solution (mg/mL)
CU = concentration of Celecoxib in the Sample solution (mg/mL)
Acceptance criteria
Individual impurities: See Table 1.
[NOTE-Disregard any impurity peak less than 0.05%.]
Table 1
| Name | Relative Retention Time | Acceptance Criteria NMT (%) |
| Celecoxib related compound Aa | 0.9 | 0.4 |
| Celecoxib | 1.0 | — |
| Celecoxib related compound Bb | 1.1 | 0.10 |
| Individual unspecified impurity | — | 0.10 |
| Total impurities | — | 0.5 |
a 4-[5-(3-Methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]benzenesulfonamide.
b 4-[3-(4-Methylphenyl)-5-(trifluoromethyl)-1H-pyrazol-1-yl]benzenesulfonamide.
SPECIFIC TESTS
• WATER DETERMINATION, Method I <921>: NMT 0.5%, using a 400-mg sample
ADDITIONAL REQUIREMENTS
• PACKAGING AND STORAGE: Preserve in tight containers, protected from light and moisture. Store at room temperature
• USP REFERENCE STANDARDS <11>
USP Celecoxib RS
p-[5-p-Tolyl-3-(trifluoromethyl)pyrazol-1-yl]benzenesulfonamide
C17H14F3N3O2S 381.4
USP Celecoxib Related Compound A RS
4-[5-(3-Methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]benzenesulfonamide.
C17H14F3N3O2S 381.4
USP Celecoxib Related Compound B RS
4-[3-(4-Methylphenyl)-5-(trifluoromethyl)-1H-pyrazol-1-yl]benzenesulfonamide.
C17H14F3N3O2S 381.4
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc.
Advantages? Superior quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
| Hazard Symbols | Xn - Harmful |
| Risk Codes | R20/21/22 - Harmful by inhalation, in contact with skin and if swallowed. |
| R52 - Harmful to aquatic organisms | |
| R61 - May cause harm to the unborn child | |
| R60 - May impair fertility | |
| Safety Description | S22 - Do not breathe dust. |
| S24/25 - Avoid contact with skin and eyes. | |
| S28 - After contact with skin, wash immediately with plenty of soap-suds. | |
| S37/39 - Wear suitable gloves and eye/face protection | |
| UN IDs | UN 3077 9 / PGIII |
| WGK Germany | 3 |
| RTECS | DB2944937 |
| HS Code | 2935900090 |
| Hazard Class | IRRITANT |
Celecoxib and Rofecoxib are two currently used COX-2 inhibitors. Successfully developed by GD Searle & Pfizer Co. (U.S.,) released in 1999, trade name: Celebrex. Celecoxib is a nonsteroidal, anti-inflammatory agent with significant analgesic and anti-inflammatory effects, causing the lowest incidence of upper gastrointestinal tract ulcers and other complications. Used clinically to treat acute and chronic osteoarthritis and rheumatoid arthritis, with an anti-inflammatory analgesic role, relieving the signs and symptoms of osteoarthritis and rheumatoid arthritis.
Celecoxib (CAS: 169590-42-5), For relief and management of osteoarthritis (OA), rheumatoid arthritis (RA), juvenile rheumatoid arthritis (JRA), ankylosing spondylitis, acute pain, primary dysmenorrhea and oral adjunct to usual care for patients with familial adenomatous polyposis.
Celecoxib (CAS: 169590-42-5) has the anti-inflammatory and analgesic effect of NSAIDs. Because of its chemical structure, it can be combined with COX-2, selectively inhibiting COX-2. Its phenyl group binds with the hydrophobic channel of COX-2, and its hydrophilic sulfonamide forms a hydrogen chain with 513 arginine and 90 histidine in the COX-2 "side pocket.” It is also in close contact with arginine in the COX-2120 position and plays a role in inhibiting COX-2 from converting arachidonic acid to prostaglandins which are harmful to the human body. Due to subtle differences between the structures of COX-1 and COX-2, Celecoxib cannot enter the COX-1 molecule, nor inhibit its transformation of arachidonic acid into prostaglandins. Thus, it has good anti-inflammatory and analgesic effects, protects gastric mucosa, protects renal blood flow, regulates platelet aggregation, and resolves the gastric irritation problems of commonly used NSAIDs.
Celecoxib (CAS: 169590-42-5) is indicated for the treatment of osteoarthritis and rheumatoid arthritis. Its use is contraindicated in individuals with hypersensitivity to sulfonamides or other NSAIDs. It should be used with caution in persons with hepatic disease. Interactions occur with other drugs that induce CYP2C9 (e.g. rifampin rifampin) or compete for metabolism by this enzyme (e.g. fluconazole, leflunomide). The most common adverse reactions to celecoxib are mild to moderate GI effects such as dyspepsia, diarrhea, and abdominal pain. Serious GI and renal effects have occurred rarely.