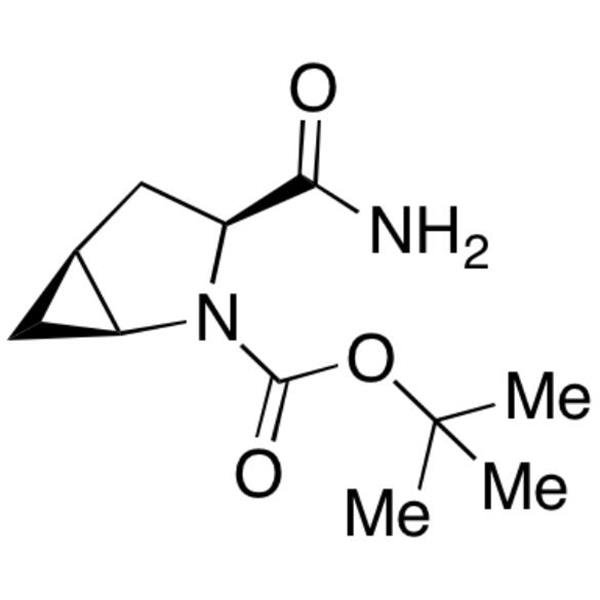
CAS 361440-67-7 Purity >98.5% (HPLC) Factory
Ruifu Chemical Supply Related Intermediates
CAS 361442-04-8
CAS 945667-22-1
(R)-(-)-3-Aminopiperidine Dihydrochloride CAS 334618-23-4
Boc-3-Hydroxy-1-Adamantyl-D-Glycine CAS 361442-00-4
CAS 361440-67-7
(R)-1-Boc-3-Aminopiperidine CAS 188111-79-7
| Chemical Name | (1S,3S,5S)-3-(Aminocarbonyl)-2-Azabicyclo[3.1.0]hexane-2-Carboxylic Acid tert-Butyl Ester |
| Synonyms | N-Boc-L-cis-4,5-Methanoprolineamide; tert-Butyl (1S,3S,5S)-3-Carbamoyl-2-Azabicyclo[3.1.0]hexane-2-Carboxylate |
| CAS Number | 361440-67-7 |
| CAT Number | RF-PI1990 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C11H18N2O3 |
| Molecular Weight | 226.27 |
| Boiling Point | 388.9±21.0℃ |
| Density | 1.228 |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | White Powder |
| Purity / Analysis Method | >98.5% (HPLC) |
| Loss on Drying | <0.50% |
| Residue on Ignition | <0.20% |
| Individual Impurities | <1.00% |
| Total Impurities | <1.50% |
| Test Standard | Enterprise Standard |
| Usage | Intermediate of API (CAS: 361442-04-8) |
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement
Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture


(1S,3S,5S)-3-(Aminocarbonyl)-2-Azabicyclo[3.1.0]hexane-2-Carboxylic Acid tert-Butyl Ester (CAS: 361440-67-7) is an intermediate of API (CAS: 361442-04-8). (CAS: 361442-04-8) is an oral hypoglycemic (anti-diabetic drug) of the dipeptidyl peptidase-4 (DPP-4) inhibitor class of drugs. Early development was solely by Bristol-Myers Squibb; in 2007 AstraZeneca joined with Bristol-Myers Squibb to co-develop the final compound and collaborate on the marketing of the drug. In June 2008, it was announced that Onglyza would be the trade name under which will be marketed. API (CAS: 361442-04-8) is used as monotherapy or in combination with other drugs for the treatment of type 2 diabetes. It is absorbed rapidly after oral administration and has a pharmacokinetic profile compatible with once daily dosing. It plays the role through inhibiting GLP-l degradation. GLP-I is the hormones naturally produced in the intestine after taking food.
-
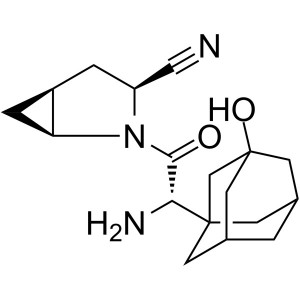
CAS 361442-04-8 Purity >99.0% (HPLC) API
-
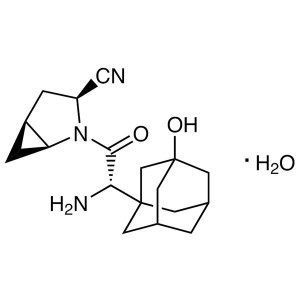
CAS 945667-22-1 Purity >99.0% (HPLC) API
-
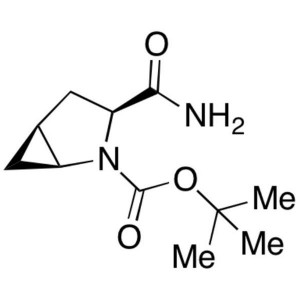
CAS 361440-67-7 Purity >98.5% (HPLC) Factory
-
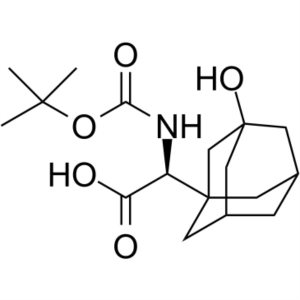
Boc-3-Hydroxy-1-Adamantyl-D-Glycine CAS 361442-...
-
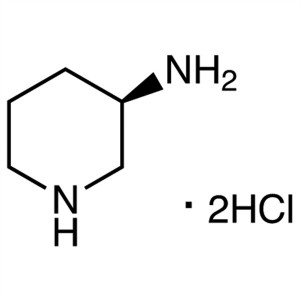
(R)-(-)-3-Aminopiperidine Dihydrochloride CAS 3...
-
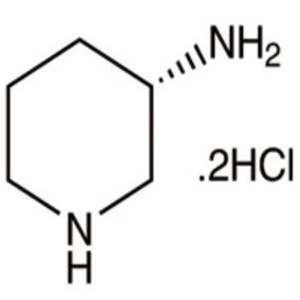
(S)-(+)-3-Aminopiperidine Dihydrochloride CAS 3...
-
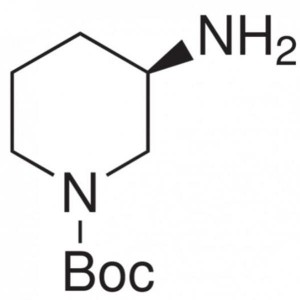
(R)-1-Boc-3-Aminopiperidine CAS 188111-79-7 Pur...
-
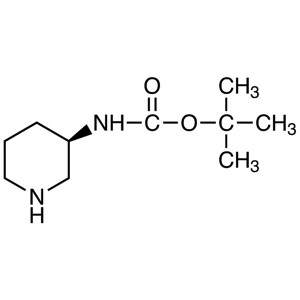
(R)-3-(Boc-Amino)piperidine CAS 309956-78-3 Lin...
-
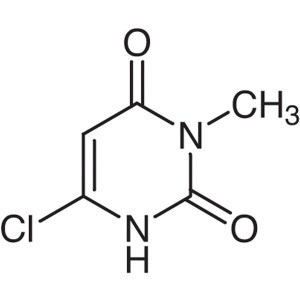
6-Chloro-3-Methyluracil CAS 4318-56-3 Purity ≥9...
-
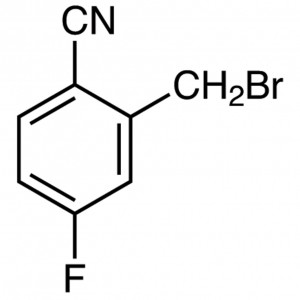
2-Cyano-5-Flurobenzyl Bromide CAS 421552-12-7 P...
-
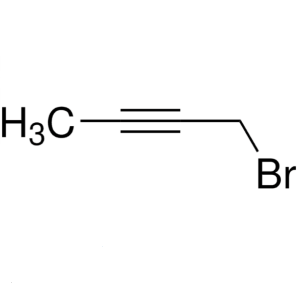
1-Bromo-2-Butyne CAS 3355-28-0 Purity ≥99.0% (G...
-
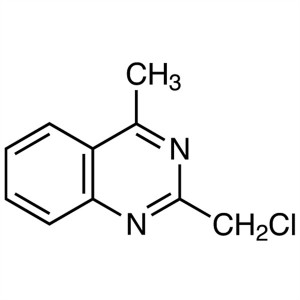
2-(Chloromethyl)-4-Methylquinazoline CAS 109113...
-
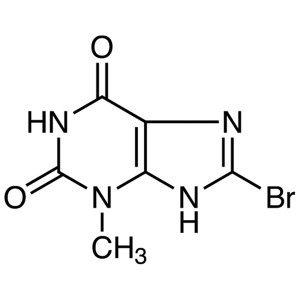
8-Bromo-3-Methylxanthine CAS 93703-24-3 Linagli...
-
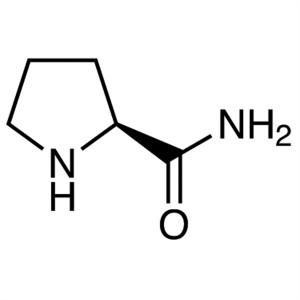
L-Prolinamide CAS 7531-52-4 (H-Pro-NH2) Purity ...
-
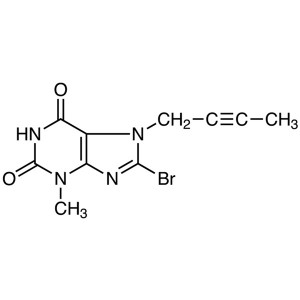
8-Bromo-7-(2-butyn-1-yl)-3-methylxanthine CAS 6...
-
Linagliptin CAS 668270-12-0 Purity ≥99.0% (HPLC...