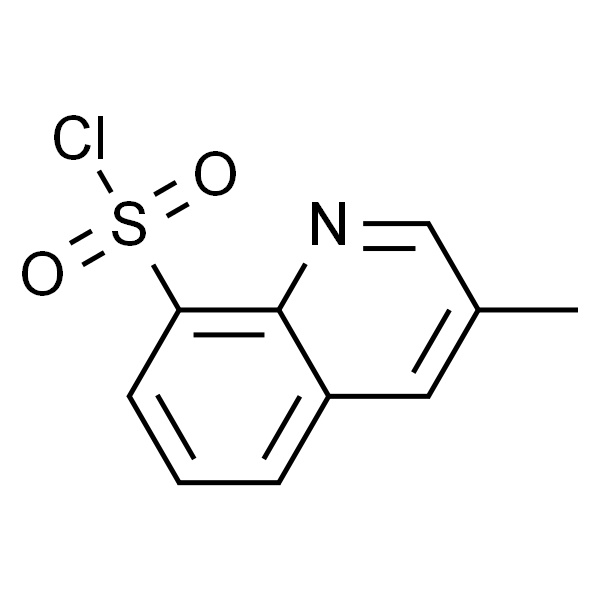
3-Methyl-8-Quinolinesulphonyl Chloride CAS 74863-82-4 Argatroban Intermediate Factory 98.0%
Manufacturer Supply Argatroban Related Intermediates:
Ethyl (2R,4R)-4-Methyl-2-Piperidinecarboxylate CAS 74892-82-3
N-Nitro-1,2,3,4-tetradehydro Argatroban Ethyl Ester CAS 74874-09-2
3-Methyl-8-Quinolinesulphonyl Chloride CAS 74863-82-4
Argatroban Monohydrate CAS 141396-28-3
Argatroban Anhydrous CAS 74863-84-6
| Chemical Name | 3-Methyl-8-Quinolinesulphonyl Chloride |
| Synonyms | 3-Methylquinoline-8-Sulfonyl Chloride |
| CAS Number | 74863-82-4 |
| CAT Number | RF-PI269 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C10H8ClNO2S |
| Molecular Weight | 241.69 |
| Density | 1.4±0.1 g/cm3 |
| Refractive Index | 1.63 |
| Solubility | Soluble in Chloroform |
| Shipping Condition | Shipped Under Ambient Temperature |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | White Crystalline Powder |
| Purity / Analysis Method | >98.0% (HPLC) |
| Melting Point | 162.0~163.0℃ |
| Loss on Drying | <0.50% |
| 3-Methylquinoline-8-Sulfonic Acid | <1.00% |
| Total Impurities | <2.00% |
| Infrared Spectrum | Conforms to Structure |
| NMR | Conforms to Structure |
| Test Standard | Enterprise Standard |
| Usage | Intermediate of Argatroban (CAS 74863-84-6) |
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement
Storage Condition: Store in sealed containers at cool and dry place; Protect from light & moisture


Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer and supplier of 3-Methyl-8-Quinolinesulphonyl Chloride (CAS: 74863-82-4) with high quality, widely used in organic synthesis, synthesis of pharmaceutical intermediates and Active Pharmaceutical Ingredient (API) synthesis. It is an intermediate typically in the synthesis of Argatroban (CAS: 74863-84-6) or Argatroban Monohydrate (CAS 141396-28-3).
Argatroban (CAS: 74863-84-6) is an anticoagulant that is a small molecule direct thrombin inhibitor. In 2000, argatroban was licensed by the Food and Drug Administration (FDA) for prophylaxis or treatment of thrombosis in patients with heparin-induced thrombocytopenia (HIT). In 2002, it was approved for use during percutaneous coronary interventions in patients who have HIT or are at risk for developing it. In 2012, it was approved by the MHRA in the UK for anticoagulation in patients with heparin-induced thrombocytopenia Type II (HIT) who require parenteral antithrombotic therapy.
-
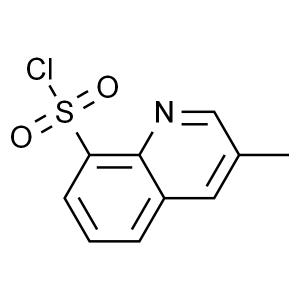
3-Methyl-8-Quinolinesulphonyl Chloride CAS 7486...
-
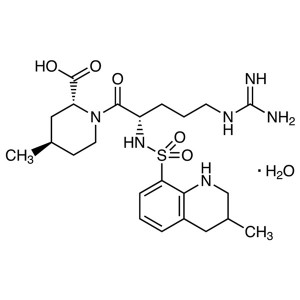
Argatroban Monohydrate CAS 141396-28-3 Purity ≥...
-
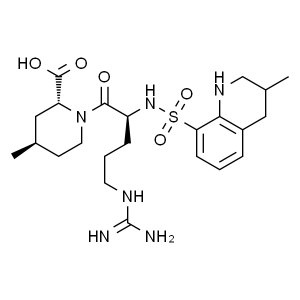
Argatroban CAS 74863-84-6 API Factory High Puri...
-
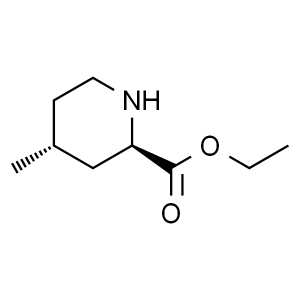
Ethyl (2R,4R)-4-Methyl-2-Piperidinecarboxylate ...
-
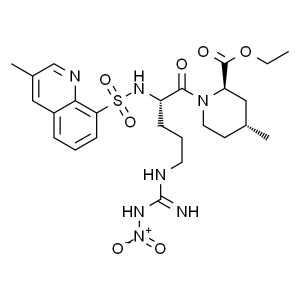
N-Nitro-1,2,3,4-tetradehydro Argatroban Ethyl E...
-
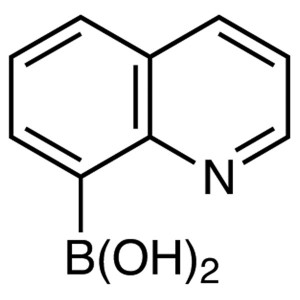
8-Quinolineboronic Acid CAS 86-58-8 Purity >98....
-
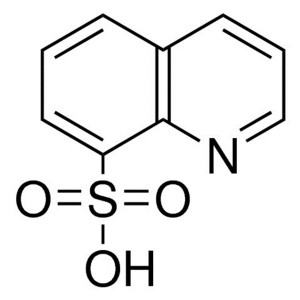
8-Quinolinesulfonic Acid CAS 85-48-3 Purity >98...
-
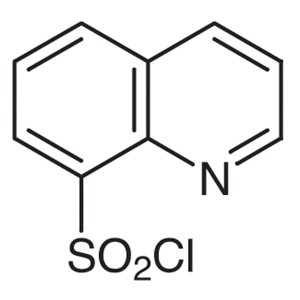
8-Quinolinesulfonyl Chloride CAS 18704-37-5 Pur...
-
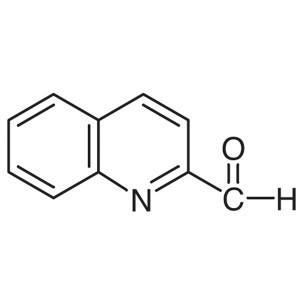
2-Quinolinecarboxaldehyde CAS 5470-96-2 Purity ...
-
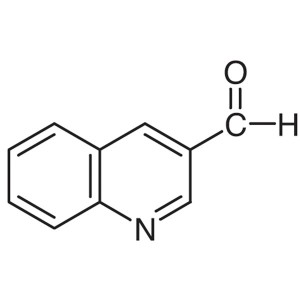
3-Quinolinecarboxaldehyde CAS 13669-42-6 Purity...
-
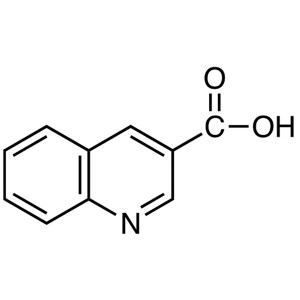
3-Quinolinecarboxylic Acid CAS 6480-68-8 Purity...
-
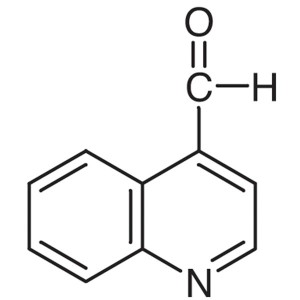
4-Quinolinecarboxaldehyde CAS 4363-93-3 Purity ...
-
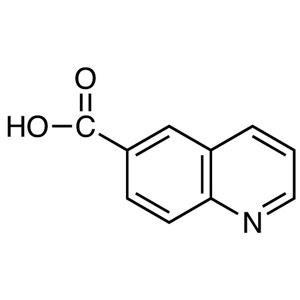
6-Quinolinecarboxylic Acid CAS 10349-57-2 Purit...
-
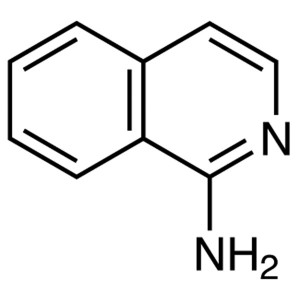
1-Aminoisoquinoline CAS 1532-84-9 Purity >99.0%...
-
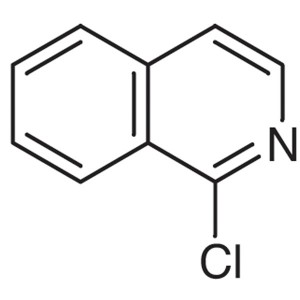
1-Chloroisoquinoline CAS 19493-44-8 Purity >98....
-
2,2′-Biquinoline CAS 119-91-5 Purity >98....